Molar weight of nitrogen
As we described in Section 4. The number of things in a mole is large, very large 6. We are all familiar with common copy-machine paper that comes in sheet reams. If you stacked up 6.
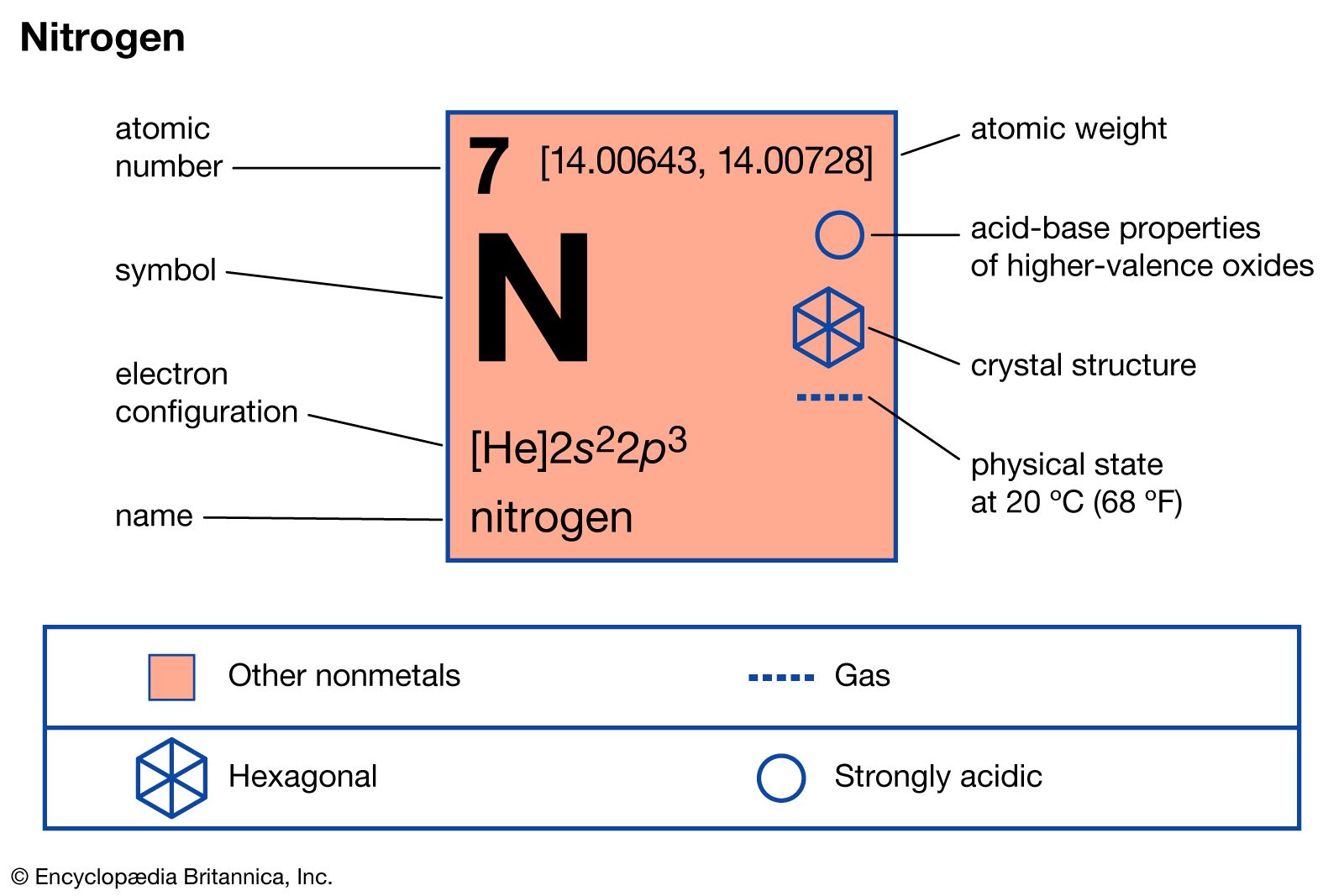
Nitrogen is a chemical element ; it has symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table , often called the pnictogens. It is a common element in the universe , estimated at seventh in total abundance in the Milky Way and the Solar System. At standard temperature and pressure , two atoms of the element bond to form N 2 , a colorless and odorless diatomic gas. Because of the volatility of nitrogen compounds, nitrogen is relatively rare in the solid parts of the Earth.
Molar weight of nitrogen
The molecular weight of a substance, also called the molar mass , M, is the mass of 1 mole of that substance, given in M gram. Molecular weight is represented by the same number in all unit systems regardless of the system used. For this reason, in many cases the unit for the molecular weight is not mentioned; however, one must realize that it is not a dimensionless parameter. The molecular weight of a pure compound is determined from its chemical formula and the atomic weights of its elements. Example: The molecular weight of ethanol C 2 H 5 OH To calculate the molecular weight of ethanol, the molecular weight of each atom in the molecule is summed:. See also Physical data for hydrocarbons , Physical data for alcohols and carboxylic acids , Physical data for organic nitrogen compounds and Physical data for organic sulfur compounds. Add standard and customized parametric components - like flange beams, lumbers, piping, stairs and more - to your Sketchup model with the Engineering ToolBox - SketchUp Extension - enabled for use with older versions of the amazing SketchUp Make and the newer "up to date" SketchUp Pro. Translate this page to Your Own Language. If you want to promote your products or services in the Engineering ToolBox - please use Google Adwords. Temperature o C K o F. Length m km in ft yards miles naut miles. Area m 2 km 2 in 2 ft 2 miles 2 acres. Volume m 3 liters in 3 ft 3 us gal. Weight kg f N lbf. Make Shortcut to Home Screen?
Hydrazine is a fuming, colourless liquid that smells similar to ammonia. Most commonly the oxygen range to alert personnel is when oxygen levels get below E numbers.
.
The purpose of the fee is to recover costs associated with the development of data collections included in such sites. Your institution may already be a subscriber. Follow the links above to find out more about the data in these sites and their terms of usage. Go To: Top , References , Notes. Data compilation copyright by the U. Secretary of Commerce on behalf of the U. All rights reserved. Acree, Jr. In addition to the Thermodynamics Research Center TRC data available from this site, much more physical and chemical property data is available from the following TRC products:.
Molar weight of nitrogen
Molar mass of Nitrogen N 2 is Then, lookup atomic weights for each element in periodic table : N: Weights of atoms and isotopes are from NIST article. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students. By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy. How to cite? Enter a chemical formula to calculate its molar mass and elemental composition:. First, compute the number of each atom in N 2 : N: 2 Then, lookup atomic weights for each element in periodic table : N: Nitrogen is a colorless, odorless gas that exists as diatomic molecules N2 in its most common form.
Carl banks jersey
Denise; Hughes, Paul S. Current Pharmaceutical Design. Bibcode : JChEd.. Beer: Quality, Safety and Nutritional Aspects. PMID Archived from the original on 14 October Because of its low cost, liquid nitrogen is often used for cooling even when such low temperatures are not strictly necessary, such as refrigeration of food, freeze-branding livestock, freezing pipes to halt flow when valves are not present, and consolidating unstable soil by freezing whenever excavation is going on underneath. It reacts with oxygen to give brown nitrogen dioxide and with halogens to give nitrosyl halides. Archived from the original on Despite it being an endothermic compound, it is kinetically stable. Nitro-nitrito isomerism is common, where the nitrito form is usually less stable. As a dilute gas it is less dangerous and is thus used industrially to bleach and sterilise flour. Generalizing this definition, the molar mass of any substance in grams per mole is numerically equal to the mass of that substance expressed in atomic mass units. Download as PDF Printable version. Elsevier BV.
Nitrogen is a chemical element ; it has symbol N and atomic number 7.
Biological Anthropology of the Human Skeleton 2nd ed. Chaptal's meaning was that nitrogen is the essential part of nitric acid , which in turn was produced from nitre. Nitrogen must first be processed, or " fixed ", into a plant-usable form, usually ammonia. Coordination Chemistry Reviews. Nitrogen is a constituent of every major pharmacological drug class, including antibiotics. Retrieved 27 September Philosophical Transactions of the Royal Society of London. It thus undergoes self-dissociation, similar to water, to produce ammonium and amide. It may be considered the conjugate acid of the azide anion, and is similarly analogous to the hydrohalic acids. The impurities can be removed by passing the gas through aqueous sulfuric acid containing potassium dichromate. English translation: Dobbin, Leonard Volume m 3 liters in 3 ft 3 us gal. Triple bonds have short bond lengths in this case, In concentrated sulfuric acid, nitric acid is protonated to form nitronium , which can act as an electrophile for aromatic nitration: [68].

It seems to me, you are not right
Quite right! Idea good, I support.