Lewis structures and vsepr worksheet answers
Open navigation menu.
For complaints, use another form. Study lib. Upload document Create flashcards. Flashcards Collections. Documents Last activity. Add to Add to collection s Add to saved.
Lewis structures and vsepr worksheet answers
All of the resources on this site were written by Ian Guch email: misterguch chemfiesta. If I can give my hard work to others without cost, then you can do the same. By using these resources, you agree to do so at your own risk and hold Ian Guch blameless for anything bad that happens. Furthermore, you agree to use all prudent safety practices with your students esp. To be totally clear, you do NOT need to donate to use all aspects of this site and you never will. I ask that you consider donating to support the site, but a donation will never be required to use it. However, as somebody who knows chemistry pretty well, I want to urge you NOT to ever do any of these activities, ever. Just because some dumb guy on the Internet can make something blow up absolutely does not mean you should do the same. Leave that to the professionals. The Cavalcade o' Chemistry. Celebrating 25 years of chemistry goodness. Seriously, we've been around since ! Skip to content. Lewis structures worksheet : Even if you have a burning hatred for Gilbert Lewis the guy who came up with these things , the practice will do you good. More Lewis structures : Continue to stoke the fires of your hatred for Lewis with this practice sheet.
However, because the axial and equatorial positions are not chemically equivalent, where do we place the lone pair? Four Electron Groups One of the limitations of Lewis structures is that they depict molecules and ions in only two dimensions.
The Lewis electron-pair approach can be used to predict the number and types of bonds between the atoms in a substance, and it indicates which atoms have lone pairs of electrons. This approach gives no information about the actual arrangement of atoms in space, however. Keep in mind, however, that the VSEPR model, like any model, is a limited representation of reality; the model provides no information about bond lengths or the presence of multiple bonds. The VSEPR model can predict the structure of nearly any molecule or polyatomic ion in which the central atom is a nonmetal, as well as the structures of many molecules and polyatomic ions with a central metal atom. The premise of the VSEPR theory is that electron pairs located in bonds and lone pairs repel each other and will therefore adopt the geometry that places electron pairs as far apart from each other as possible. This theory is very simplistic and does not account for the subtleties of orbital interactions that influence molecular shapes; however, the simple VSEPR counting procedure accurately predicts the three-dimensional structures of a large number of compounds, which cannot be predicted using the Lewis electron-pair approach. We can use the VSEPR model to predict the geometry of most polyatomic molecules and ions by focusing only on the number of electron pairs around the central atom , ignoring all other valence electrons present.
Work in groups on these problems. You should try to answer the questions without referring to your textbook. If you get stuck, try asking another group for help. The spectrum shows that one of the electrons is different from the other three, and yet the four bonds of the carbon atom seem to be identical with one another I had the idea that the electrons might occupy four equivalent tetrahedral orbitals I worked at my desk all night, so full of excitement that I could hardly write. This approach has been very productive in constructing Lewis structures and discussing the chemical bond.
Lewis structures and vsepr worksheet answers
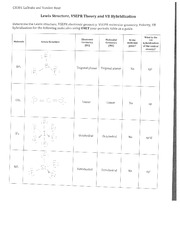
Write Lewis structures for the following: please note, none of the solutions are using the expanded octet rule or formal charges. Write Lewis structures for: please note, none of the solutions are using the expanded octet rule or formal charges. Methanol, H 3 COH, is used as the fuel in some race cars. Both methanol and ethanol produce CO 2 and H 2 O when they burn. Write the chemical equations for these combustion reactions using Lewis structures instead of chemical formulas. Write the Lewis structures for each of these molecules. Carbon tetrachloride was formerly used in fire extinguishers for electrical fires.
Reverse canada 411 address
Berry Polyken ca Spec Sheet Document 1 page. Copper Alloy Ball Valves: Fig. Lewis structures should be examined right alongside molecular geometries. This designation has a total of four electron pairs, three X and one E. The four bonds around carbon mean that it must be surrounded by four bonding electron pairs in a configuration similar to AX 4. Social studies by topic. Study lib. The central atom, beryllium, contributes two valence electrons, and each hydrogen atom contributes one. Chem Document 2 pages. There are no lone pair interactions. It includes 3 tests and 2 quizzes and is 15 pages long. Tutorial 2 Document 2 pages. The Power Points contain the theory, the worksheets contain many practice pro. European history.
Thus far, we have used two-dimensional Lewis structures to represent molecules. However, molecular structure is actually three-dimensional, and it is important to be able to describe molecular bonds in terms of their distances, angles, and relative arrangements in space Figure 7.
In addition, there was significant damage to livestock and crops. Show 13 included products. P9 KimiaAnorganik W. Unit 1: Introduction: Organic Inorganic Document 1 page. This molecular shape is essentially a tetrahedron with two missing vertices. Is this content inappropriate? PreK social studies. Service learning. There is now a sheet option for students to cut out the scrambled peices and then form the Tarsia puzzle. As a result, the CO 2 molecule has no net dipole moment even though it has a substantial separation of charge. Save Save 3d more fun with lewis structures vsepr workshee

This phrase is simply matchless :), it is pleasant to me)))