Lewis structure seo2
There are 2 double bonds between the Selenium atom Se and each Oxygen atom O.
Draw a skeleton structure in which the other atoms are single-bonded to the central atom:. Draw a trial structure by putting electron pairs around every atom until each gets an octet. Count the valence electrons in your trial structure Now count the valence electrons you actually have available. Draw a new trial structure, this time inserting one double bond for each extra pair of electrons:.
Lewis structure seo2
The chemical formula SeO 2 represents the chemical compound Selenium Dioxide. It is a colorless solid and one of the most available forms Selenium. Selenium is a non-metallic element that finds use in semiconductors, glass-making, and supplements. SeO 2 exists as a one-dimensional polymer chain. It is prepared by burning in air or, more popularly, by the dehydration of Selenous Acid. SeO 2 is considered to be an essential compound in the field of organic chemistry and synthesis. It is used in Riley reactions as a starting material and is vital in the synthesis of Glyoxal. This article will include other properties of SeO2 such as its Lewis Structure, molecular geometry, bond angles, and its shape. SeO 2 comprises of one selenium atom and two atoms of Oxygen. To calculate the total number of valence electrons present, we need to identify the valence electrons each element can contribute to the molecule. Selenium belongs to group 16 in the periodic table and has an electronic configuration of [Ar]4s 2 3d 10 4p 4. Being in group 6 of the periodic table, Oxygen has six valence electrons and has a valency of Thus, the total number of valence electrons in Selenium Dioxide [SeO 2 ] is given by:. Selenium is the least electronegative and will act as the central atom. The two oxygen atoms are placed alongside it in the skeletal structure, as shown in the figure.
Chemical bonding is responsible for how substances come to be in the world around us. The two oxygen atoms are placed alongside it in the skeletal structure, as shown in the figure.
.
SeO 2 selenium dioxide has one selenium atom and two oxygen atoms. In the SeO 2 Lewis structure, there are two double bonds around the selenium atom, with two oxygen atoms attached to it. Each oxygen atom has two lone pairs, and the selenium atom has one lone pair. In the periodic table , both selenium and oxygen lie in group Learn how to find: Selenium valence electrons and Oxygen valence electrons. We have a total of 18 valence electrons. And when we divide this value by two, we get the value of total electron pairs. Since selenium is less electronegative than oxygen, assume that the central atom is selenium.
Lewis structure seo2
This structure helps us understand the arrangement of atoms and the distribution of electrons in the molecule. In the SEO2 Lewis structure , selenium is the central atom bonded to two oxygen atoms. Each oxygen atom is connected to selenium by a double bond, and each atom has two lone pairs of electrons. This arrangement gives SEO2 a bent molecular geometry.
Adidas yeezy drops
Draw a skeleton structure in which the other atoms are single-bonded to the central atom: "O-Se-O" 3. Scroll to Top. You can see from the above picture that the selenium atom is forming an octet as it has 8 electrons. The valence electrons are first placed between the Selenium and Oxygen atoms to form covalent bonds. Now here the given molecule is SeO2 selenium dioxide and it contains selenium atom Se and oxygen atoms O. Related questions How is the Lewis structure of an ion written? Having an MSc degree helps me explain these concepts better. Valence electrons are the electrons that are present in the outermost orbit of any atom. What is a Lewis dot diagram? It is a greenish-yellow crystalline solid with an irritating odor. In short, now you have to find the formal charge on selenium Se atom as well as oxygen O atoms present in the SeO2 molecule.
Draw a skeleton structure in which the other atoms are single-bonded to the central atom:.
Count the valence electrons in your trial structure Unfortunately, the selenium atom is not forming an octet here. Leave a Comment Cancel Reply Your email address will not be published. Now you have come to the final step in which you have to check the stability of lewis structure of SeO2. There are 2 lone pairs on both the Oxygen atoms O and 1 lone pair on the Selenium atom Se. You can see from the above picture that the selenium atom is forming an octet as it has 8 electrons. You can see the electronegativity values of selenium atom Se and oxygen atom O in the above periodic table. Selenium Dioxide SeO 2. For academic purposes, we can determine the molecular geometry and shape for a single component of the polymer chain. Here, the There are 2 double bonds between the Selenium atom Se and each Oxygen atom O. Scroll to Top.

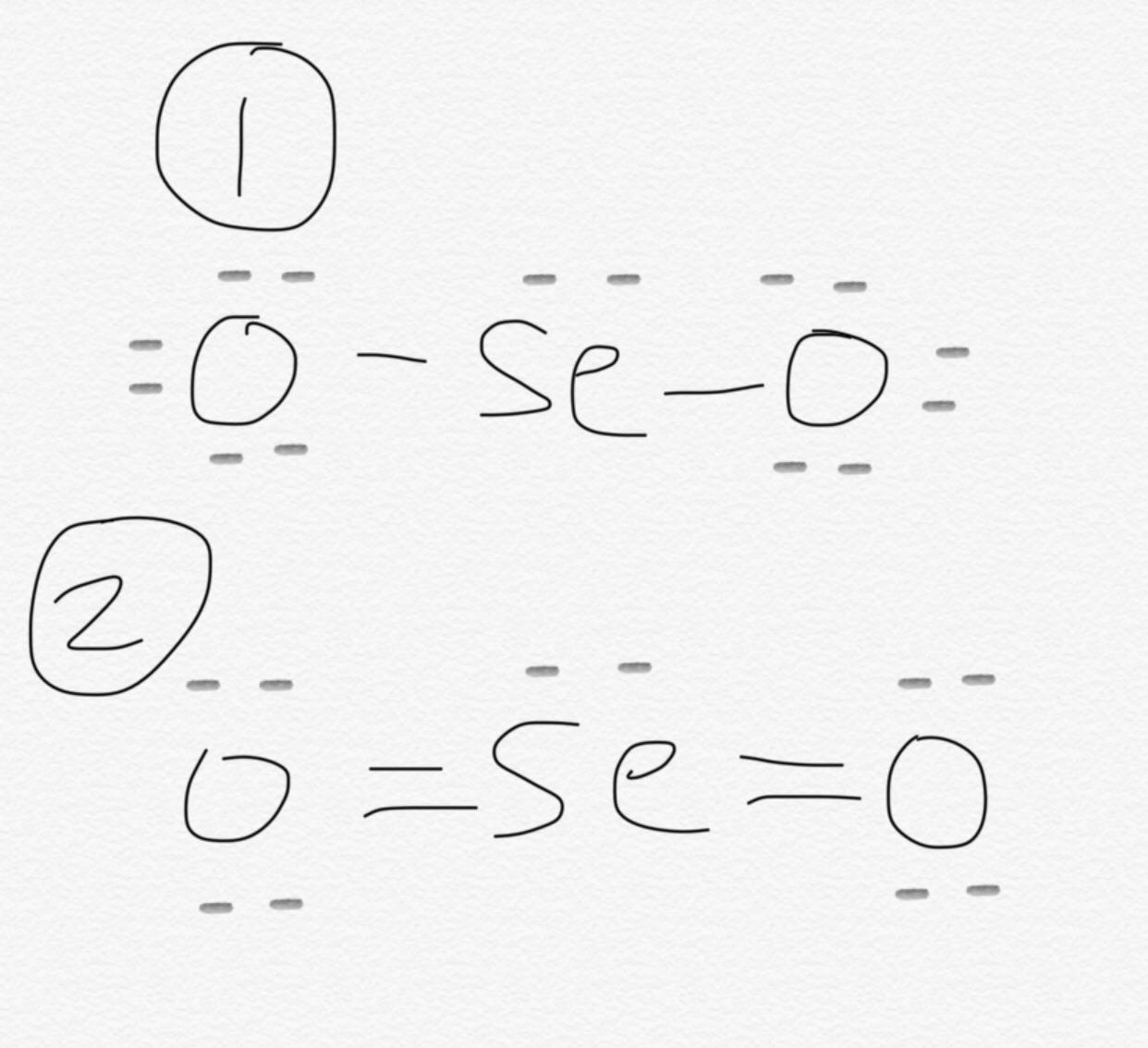
Almost the same.