Lewis structure of sf6
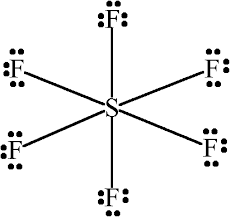
Sulfur atom S is the central atom, fluorine atom F is the external atom, sulfur atom S and each fluorine atom F are connected by a single bond, each fluorine atom F has three lone pairs of electrons, and the central atom is symmetrically distributed around. The SF6 bond angle is 90 degrees. The SF6 Lewis structure is shown below:. Based on the information in the periodic table, lewis structure of sf6, we are able to obtain: Sulphur S and fluorine F are in the 16th and lewis structure of sf6 group of the periodic table.
This article is about the SF6 Lewis Structure, the Molecular geometry, and the formal charge present in the molecule. A Lewis Structure is a graphical representation of the valence shell electrons of a molecule. The Lewis structure was initially proposed by famous scientist Gilbert N. The Lewis structure is important in chemistry because it can predict the number of bonds, nonbonding electrons, and bonding electron structure. Lewis structure does not try to explain the molecular shape, bond formation, or electron sharing between atoms. It is the most basic and limiting explanation of the electrical structure.
Lewis structure of sf6
Draw the Lewis structure of HCN. Draw the Lewis structure of B e C l 2. Draw the Lewis structure of C l O 4 per chlorate ion. Write the Lewis dot structure of C O molecule. Draw the Lewis structure of nitric acid, H N O 3. Draw the Lewis structure for SF6. Which one of the following molecules contains no pi - bond? Which of the following is a polar moleule? Which of the following is paramagnetic? According to MO theory which of thhe following lists makes the nitroge In the case of alkali metals, the covalent character decreases in the The state of hybridization of C2, C3, C5, and C6 of the hydrocarbon
It is also used as a silicon etchant in semiconductor fabrication and as an inert gas in magnesium casting. Draw the Lewis structure of C l O 4 per chlorate ion. View Solution.
.
In this tutorial, we will illustrate the step-by-step process of drawing the Lewis structure for sulfur hexafluoride SF6. SF6 is a molecular compound composed of sulfur and fluorine, both non-metals, indicating a sharing of electrons in its structure. Determine Total Valence Electrons. To begin, we need to determine the total number of valence electrons in the molecule. Sulfur, found in group 16 of the periodic table, contributes six valence electrons, while each of the six fluorine atoms from group 17 brings seven valence electrons. The sum of these yields 48 valence electrons for SF6. The next step involves placing the central atom, which is usually less electronegative. In this case, sulfur, which can form up to six bonds, is chosen as the central atom. The six fluorine atoms are then arranged around sulfur, and single bonds are initially drawn between the central sulfur and each surrounding fluorine.
Lewis structure of sf6
In all cases, these bonds involve the sharing or transfer of valence shell electrons between atoms. In this section, we will explore the typical method for depicting valence shell electrons and chemical bonds, namely Lewis symbols and Lewis structures. We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons:. Figure 1 shows the Lewis symbols for the elements of the third period of the periodic table. Lewis symbols can also be used to illustrate the formation of cations from atoms, as shown here for sodium and calcium: Likewise, they can be used to show the formation of anions from atoms, as shown below for chlorine and sulfur: Figure 2 demonstrates the use of Lewis symbols to show the transfer of electrons during the formation of ionic compounds. We also use Lewis symbols to indicate the formation of covalent bonds, which are shown in Lewis structures , drawings that describe the bonding in molecules and polyatomic ions. For example, when two chlorine atoms form a chlorine molecule, they share one pair of electrons:. The Lewis structure indicates that each Cl atom has three pairs of electrons that are not used in bonding called lone pairs and one shared pair of electrons written between the atoms.
Interpals penpals
In the case of alkali metals, the covalent character decreases in the Four diatomic species are listed in different sequence. The hybridization of oxygen atom in H2O2 is Sulfur hexafluoride manufacturers. In the SF6 molecule, the total number of electrons in the sulphur is 16, so the shell layer is populated with different energy levels depending on its capacity and level of hierarchy. Which one of the following pairs consists of only paramagnetic species Factors affecting polarity. Hybridization of SF6. Based on the information in the periodic table, we are able to obtain: Sulphur S and fluorine F are in the 16th and 17th group of the periodic table. The most significant aspects of being the center atom are having a high valence and being the most electropositive atom. Draw the Lewis structure of nitric acid, H N O 3. Geranyl linalool, a versatile terpene, is widely used in fragrances and cosmetics due to its safety and pleasant aroma Some Other Uses of SF6.
SF 6 sulfur hexafluoride has one sulfur atom and six fluorine atoms. In the SF 6 Lewis structure, there are six single bonds around the sulfur atom, with six fluorine atoms attached to it, and on each fluorine atom, there are three lone pairs.
In which of the following pairs are the two species isostructural? This creates six hybrid orbitals one 3s, three 3p and two 3d. These six orbitals are located in the six directions of the octahedral shape. What is the molecular geometry of SF6? The center atom, on the other hand, binds with six Fluorine atoms, giving SF6 its octahedral structure. Geranyl linalool: properties, applications and toxicity. The dipole moment is cancelled due to the symmetric configuration. SF6 is polar or non-polar? Sulfur Hexafluoride is a nonpolar molecule and different factors can affect the polarity of the molecule which include electronegativity, dipole moment, and the molecular geometry of the molecule. Lewis structure of any molecule is important because.

From shoulders down with! Good riddance! The better!