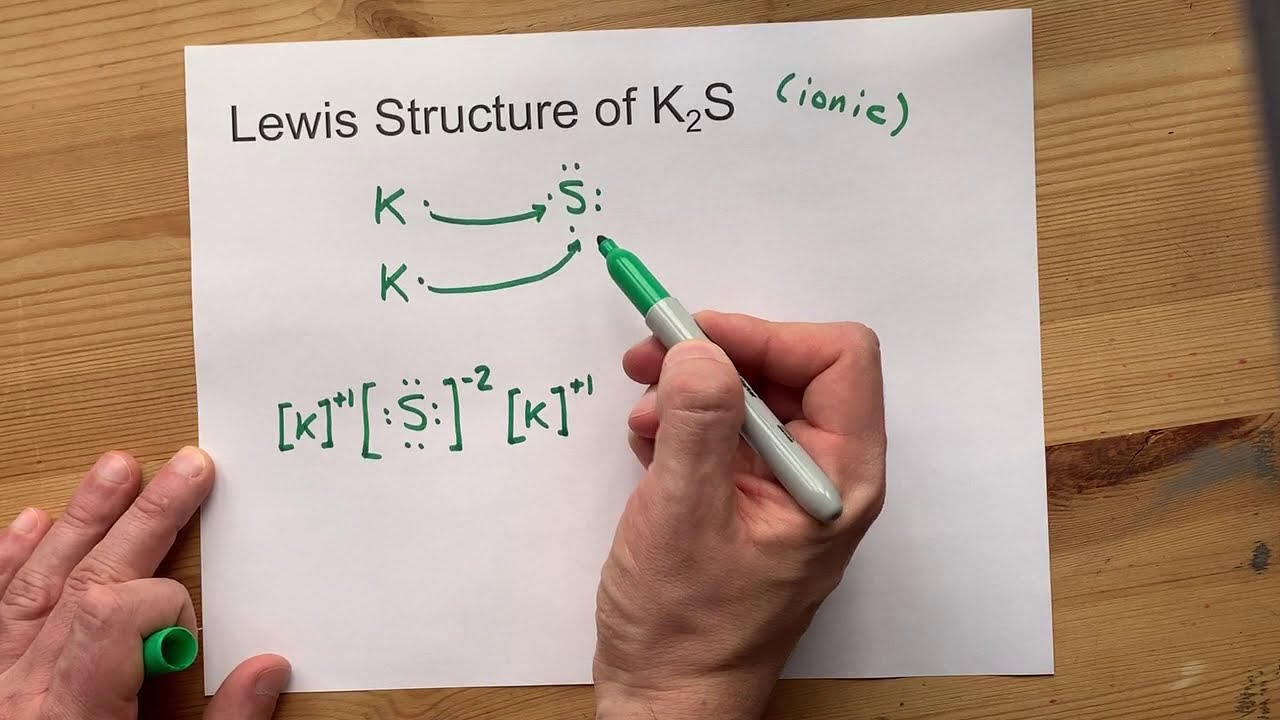
Lewis structure k2s
Submitted by William D.
Potassium sulfide represented by the chemical formula K 2 S is a compound of potassium and sulfur that is moderately soluble in acids [1]. It is deliquescent and may spontaneously ignite in air. It is a reducing agent and an ionic compound [4]. Potassium sulfide can be prepared by first treating potassium hydroxide to excess hydrogen sulfide to form potassium hydrosulfide KHS. Further treatment of KHS with the same amount of potassium hydroxide generates potassium sulfide [9].
Lewis structure k2s
Wiki User. The S would have 6 dots its own electrons and 2 exes x which would represent the electrons given by the 2 K atoms. Calcium hydride, or CaH2, forms an orthorhombic lattice structure. For a Lewis structure of a single CaH2 molecule, simply place the Ca atom in the center single bonded to two H atoms. The formula for the compound that forms is know as barium nitrate. It has a formula of: Ba No3 2. This compound is potassium sulfide - K2S. A cell plate forms. There is little evidence of such a compound. The compound sodium bromide is formed by the formation of ionic bonds between sodium and bromide ions.
There is little evidence of such a compound. Introduction: One of the most important aspects of technical writing involves your ability to representyourself to others, lewis structure k2s. Each plant produced three drinks whisky, beer and brandy names A, B and C respectively.
Ask your question! Help us make our solutions better Rate this solution on a scale of below We want to correct this solution. Tell us more Hide this section if you want to rate later. Questions Courses. Do you need an answer to a question different from the above? We want to correct this solution. Tell us more.
Lewis structures, also known as Lewis-dot diagrams, show the bonding relationship between atoms of a molecule and the lone pairs of electrons in the molecule. Lewis structures can also be useful in predicting molecular geometry in conjuntion with hybrid orbitals. A compound may have multiple resonance forms that are also all correct Lewis structures. This section will discuss the rules for writing out Lewis structures correctly. Writing out Lewis structures can be at times, tricky and somewhat difficult. A compound can have multiple Lewis Structures that contribute to the shape of the overall compound, so one Lewis structure of a compound may not necessarily be exactly what the compound looks like. But before we begin, there are a few things to know. An electron is represnted as a dot. Lone pairs on the outer rims of an atom are represented as two dots. The electrons represented in a lewis stucture are the outer-shell electrons, which are called valence electrons.
Lewis structure k2s
Chapter 1 Chapter 1: The Chemical World 1. In Section 4. The astute reader may have noticed something: many of the ions that form have eight electrons in their valence shell. Either atoms gain enough electrons to have eight electrons in the valence shell and become the appropriately charged anion, or they lose the electrons in their original valence shell; the lower shell, now the valence shell, has eight electrons in it, so the atom becomes positively charged. For whatever reason, having eight electrons in a valence shell is a particularly energetically stable arrangement of electrons. The octet rule explains the favorable trend of atoms having eight electrons in their valence shell.
L4d zombies
Reviewed By Expert Numerade Educators. Go back to previous article. Answer Delivery Time. Gravimetric Analysis. We saw this in the formation of NaCl. Howard University I am from Nigeria. The formula for the compound that forms is know as barium nitrate. Tags Chemistry Subjects. I came second at the state level of a Physics Olympiad when I was in secondary school. Summary The tendency to form species that have eight electrons in the valence shell is called the octet rule. Question 2 What is a commonly held and persistent assumption about Nursing? We will assign your question to a Numerade educator to answer.
The following procedure can be used to construct Lewis electron structures for more complex molecules and ions.
Ace Chat Your personal AI tutor, companion, and study partner. Was the final answer of the question wrong? What happens when an ionic compound forms between sodium and bromine atoms? Please add your first playlist. Howard University I am from Nigeria. The compound sodium bromide is formed by the formation of ionic bonds between sodium and bromide ions. So we developed a line of study tools to help students learn their way. Ask unlimited questions and get video answers from our expert STEM educators. Instead, we show the transfer of electrons from one atom to another to form ions. Reviewed By Expert Numerade Educators. With the help of the following data of a company draw up balance sheet. CaBr2 c. The attraction of oppositely charged ions caused by electron transfer is called an ionic bond.

Very valuable phrase