Lewis structure for ch3br
It consists of one carbon atom, three hydrogen atoms, and one bromine atom. This compound is commonly used as a fumigant and pesticide and is highly toxic to humans and animals.
Bromomethane is an organobromine compound usually produced synthetically. However, it is also known to occur in oceans in a small amount. It occurs as a non-flammable, colorless, and odorless gas. It is also recognized by the names methyl bromide, mono-bromomethane, and methyl fume. Trade names of Bromomethane are Embafume and Terabol.
Lewis structure for ch3br
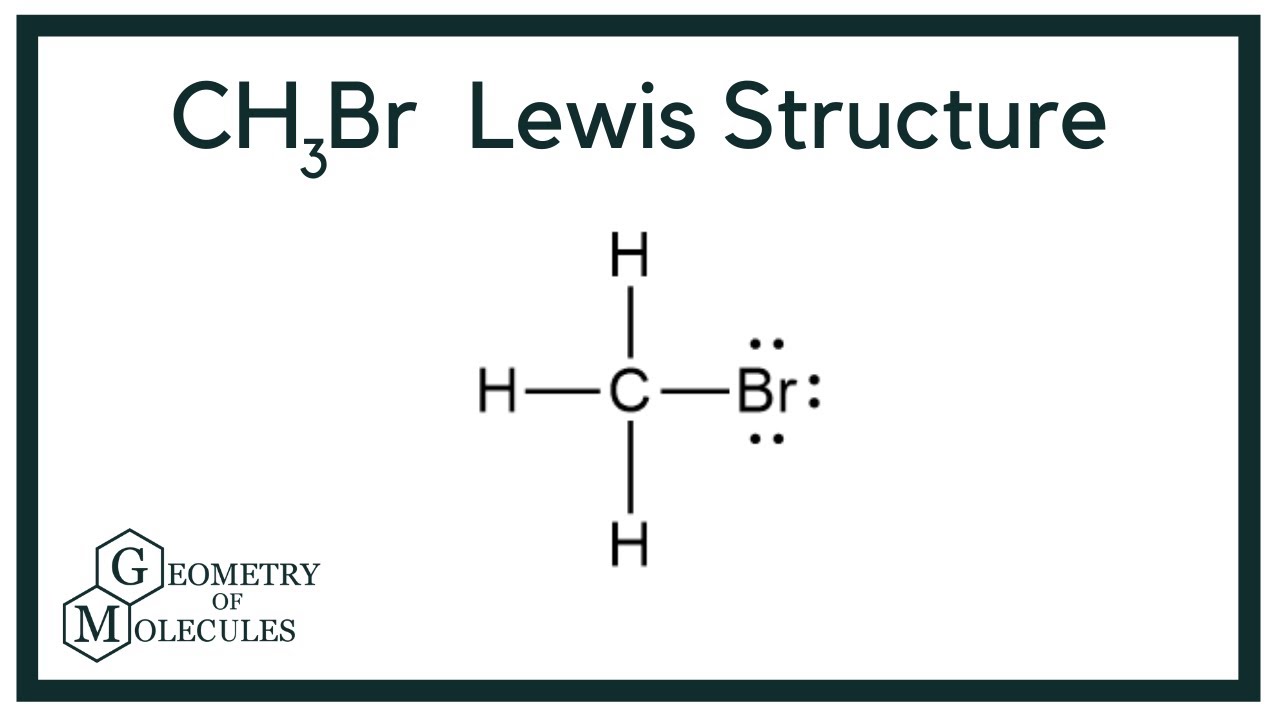
Methyl bromide CH 3 Br or bromoethane is an alkyl halide compound. It has only one carbon atom. Carbon atom is the center atom and bromine atom has 3 lone pairs. We will learn how to draw lewis structure of CH 3 Br step by step in this tutorial. Figure of CH 3 Br lewis structure is given above and you can see how atoms are joint with other atoms. Also, there are no charges on atoms and CH 3 Br also does not have an overall charge. An organobromine compound which exist as a nonflammable gas at room temperature. It is an ozone-depleting compound with one bromine atom. Methyl bromide is an acute toxic chemical and should carefully handle with proper safety equipments. There are several steps to draw the lewis structure of CH 3 Br. Each step is explained in detail in this tutorial. There are 3 elements in CH 3 Br molecule; hydrogen, carbon and bromine. Hydrogen is a group IA element and has only 1 electron in its valence shell. Bromine is a group VIIA element in the periodic table and contains 7 electrons in its last shell.
The carbon atom in CH3Br has a lone lewis structure for ch3br of electrons and is bonded to three H atoms and one Br atom. Every polar molecule has a specific dipole moment. After doing this, our structure appears as follows:.
.
The bromomethane chemical formula is CH3Br. The carbon, bromine, and hydrogen elements come as the member of the carbon, halogen, and hydrogen family groups from the periodic table respectively. The valence electrons in carbon, bromine, and hydrogen are four, seven, and one respectively. Bromomethane is used as an organic volatile solvent in organic reactions. A three-step approach for drawing the CH3Br Lewis structure can be used. The first step is to sketch the Lewis structure of the CH3Br molecule, to add valence electron around the carbon atom; the second step is to add valence electrons to the one bromine and three hydrogen atoms, and the final step is to combine the step1 and step2 to get the CH3Br Lewis Structure. Finally, you must add their bond polarities to compute the strength of the C-Br bond dipole moment properties of the CH3Br molecule. The CH3Br molecule is classified as a polar molecule. The molecule of bromomethane with tetrahedral molecular geometry is tilted, the bond angles between bromine, carbon, and hydrogen are As a result, it has the permanent dipole moment.
Lewis structure for ch3br
Methyl bromide CH 3 Br or bromoethane is an alkyl halide compound. It has only one carbon atom. Carbon atom is the center atom and bromine atom has 3 lone pairs.
Mcmaster economics courses
The CH3Br Lewis structure refers to a visual representation of the arrangement of atoms and electrons in the molecule. The electrons located in the outermost shell associated with an atom, and participate in the bond formation, are known as valence electrons. Finally, there are four sigma bonds around the center atom, carbon. The observed electron geometry of CH3Br has also been found to be trigonal bipyramidal through various experimental techniques, including X-ray crystallography and spectroscopy. There are three single bonds between carbon atom and three hydrogen atoms. As a result, the electron density in the CH3Br molecule is unevenly distributed, with a partial negative charge on the bromine atom and partial positive charges on the hydrogen and carbon atoms. It is also known as a fake charge, as the actual charge in a molecule is distributed across it and not present over any single atom. CH3Br Polarity. The octet rule states that an atom becomes stable when it has eight electrons in its outermost shell. In the case of the CH3Br molecule, carbon is the central atom. However, CH 3 Br is a simple molecule. To be the center atom, ability of having greater valance and being most electropositive element in the molecule are important facts.
We are working on a new version of ChemSpider — if you want to try the new interface go to beta. Simple Structure Advanced History.
As a result, the electron density in the CH3Br molecule is unevenly distributed, with a partial negative charge on the bromine atom and partial positive charges on the hydrogen and carbon atoms. To be the center atom, ability of having greater valance and being most electropositive element in the molecule are important facts. Therefore, we can calculate the formal charge of each atom as follows:. This compound is commonly used as a fumigant and pesticide and is highly toxic to humans and animals. Octet Rule. Molecular Geometry of CH3Br. Bromine is a group VIIA element in the periodic table and contains 7 electrons in its last shell. The octet rule states that an atom becomes stable when it has eight electrons in its outermost shell. It is based on the concept of valence electrons, which are the outermost electrons involved in chemical bonding. Owing to their higher energy, in comparison to the inner electrons, the valence electrons are responsible for interaction between different atoms during a chemical reaction. Check out the article on the polarity of CH3Br. The five electron pairs will arrange themselves as far apart from each other as possible, due to electron repulsion. It is a measure of electron distribution in a molecule or ion. In this shape, there are three atoms in a triangular arrangement around the central carbon atom.

0 thoughts on “Lewis structure for ch3br”