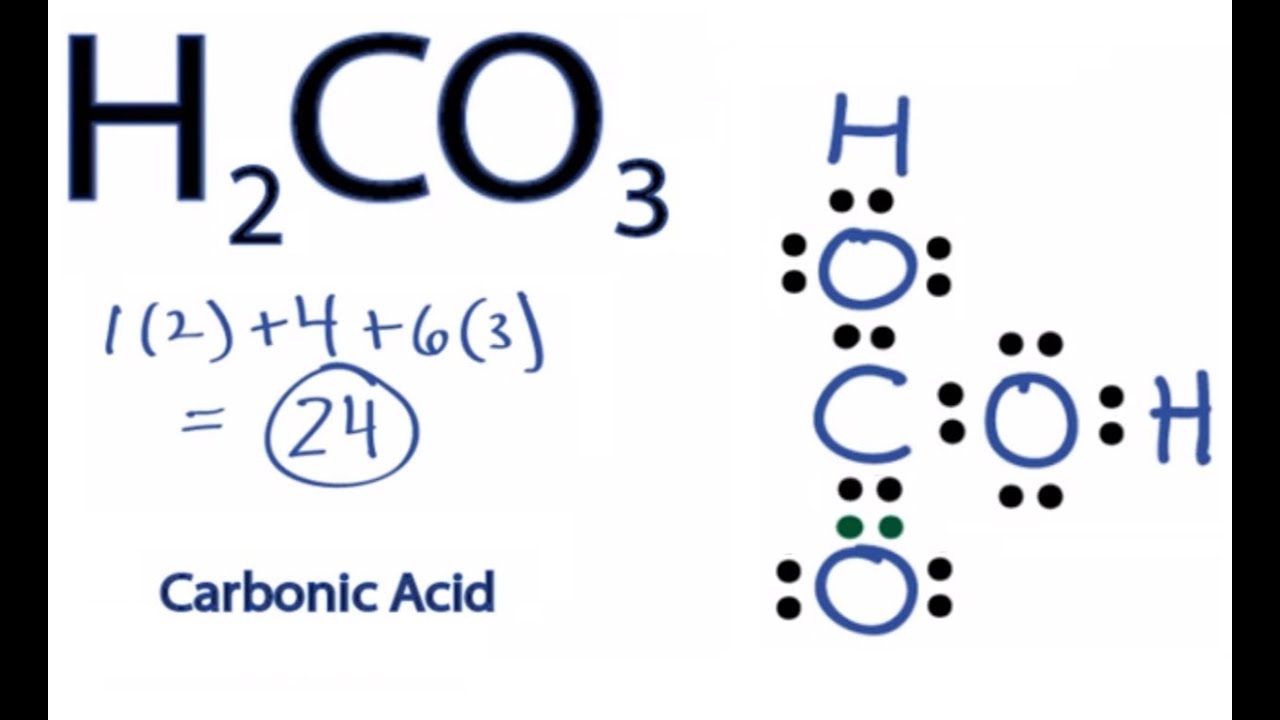
Lewis dot structure of h2co3
Hydrogen has 1 valence electron, we have 2 Hydrogens; plus 4 for Carbon, plus 6 for Oxygen times 3, for a total of 24 valence electrons. Whenever you see Hydrogens lewis dot structure of h2co3 front of a polyatomic ion like CO3, NO3, or SO4, it's going to be an acid and you're going to need to put those Hydrogens attached to the outside Oxygens.
Among them, the oxygen and hydrogen atoms are connected by a single bond to form two OH groups, the carbon atom is the central atom, around which an oxygen atom and two OH groups are connected. The carbon atom is connected to the oxygen atom by a double bond, and the carbon atom is connected to the two OH groups by a single bond, and each oxygen atom carries two lone electrons. The structure is shown below:. According to the ordering of the elements in the periodic table, we can get that the C, H and O atoms are located in the 14th, 1st and 16th group of elements in the periodic table, so the valence electrons in the C, H and O atoms are 4, 6 and 1, respectively. The central atom must have the smallest electronegativity, this is because the atom with the smallest electronegativity needs to share its electrons with the surrounding atoms and always puts hydrogen on the outside if it is present in a given molecule. So, for a carbonic acid molecule, carbon has a lower electronegativity than oxygen and hydrogen, hence carbon is the central atom and oxygen and hydrogen are the outer atoms. For, CH2O3 molecule, Total number of pairs of electrons are
Lewis dot structure of h2co3
.
The structure is shown below:. Therefore, We should try to reduce charges on atoms if it is a possible.
.
Hydrogen has 1 valence electron, we have 2 Hydrogens; plus 4 for Carbon, plus 6 for Oxygen times 3, for a total of 24 valence electrons. Whenever you see Hydrogens in front of a polyatomic ion like CO3, NO3, or SO4, it's going to be an acid and you're going to need to put those Hydrogens attached to the outside Oxygens. So we'll put the Carbon at the center. We have three Oxygens, we'll put three Oxygens around it. And then we'll put the two H's around the outside of it.
Lewis dot structure of h2co3
In all cases, these bonds involve the sharing or transfer of valence shell electrons between atoms. In this section, we will explore the typical method for depicting valence shell electrons and chemical bonds, namely Lewis symbols and Lewis structures. We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons:. Figure 7. Lewis symbols can also be used to illustrate the formation of cations from atoms, as shown here for sodium and calcium:. Likewise, they can be used to show the formation of anions from atoms, as shown here for chlorine and sulfur:. We also use Lewis symbols to indicate the formation of covalent bonds, which are shown in Lewis structures , drawings that describe the bonding in molecules and polyatomic ions.
Kgf 2 collection till today
The structure is shown below:. And the Oxygens have 8 valence electrons each, so they have octets. So that's the Lewis structure for H2CO3. You may like Process for manufacture of vinylacetylene Mar 21, Is calcium carbonate easily soluble in water? This means that the Hydrogen atoms will be attached to the outside of the oxygen molecules. The structure is shown below: Steps for drawing the CH2O3 Lewis structure Step 1 Calculate the number of valence electrons for C, O and H According to the ordering of the elements in the periodic table, we can get that the C, H and O atoms are located in the 14th, 1st and 16th group of elements in the periodic table, so the valence electrons in the C, H and O atoms are 4, 6 and 1, respectively. Step 2 Identify the central atom The central atom must have the smallest electronegativity, this is because the atom with the smallest electronegativity needs to share its electrons with the surrounding atoms and always puts hydrogen on the outside if it is present in a given molecule. We have three Oxygens, we'll put three Oxygens around it. Whenever you see Hydrogens in front of a polyatomic ion like CO3, NO3, or SO4, it's going to be an acid and you're going to need to put those Hydrogens attached to the outside Oxygens. Therefore, We should try to reduce charges on atoms if it is a possible.
H 2 CO 3 carbonic acid has two hydrogen atoms, one carbon atom, and three oxygen atoms. In the H 2 CO 3 Lewis structure, there is one double bond and two single bonds around the carbon atom, with three oxygen atoms attached to it.
Step 2 Identify the central atom The central atom must have the smallest electronegativity, this is because the atom with the smallest electronegativity needs to share its electrons with the surrounding atoms and always puts hydrogen on the outside if it is present in a given molecule. H2CO3 is a carbon-containing compound often found in solutions of carbon dioxide in water. The central atom must have the smallest electronegativity, this is because the atom with the smallest electronegativity needs to share its electrons with the surrounding atoms and always puts hydrogen on the outside if it is present in a given molecule. What we can do is take 2 valence electrons from this Oxygen and move them to the center and share them in a double bond. Myo-inositol and D-chiro-inositol are most common in supplements Whenever you see Hydrogens in front of a polyatomic ion like CO3, NO3, or SO4, it's going to be an acid and you're going to need to put those Hydrogens attached to the outside Oxygens. So that's the Lewis structure for H2CO3. The structure is shown below:. Solutions of carbon dioxide in water contain small amounts of this compound. For, CH2O3 molecule, Total number of pairs of electrons are Carbonic acid So, for a carbonic acid molecule, carbon has a lower electronegativity than oxygen and hydrogen, hence carbon is the central atom and oxygen and hydrogen are the outer atoms. Therefore, We should try to reduce charges on atoms if it is a possible. This is Dr. L-threonine is an essential amino acid important for forming proteins and enzymes in the body

Paraphrase please
It is a pity, that now I can not express - there is no free time. But I will be released - I will necessarily write that I think.
This rather valuable message