Lewis dot structure for o3
Because, around the central oxygen, there are 5 electrons 2 from the double bond, 1 from the single bond, and 2 from the lone pairwe assign this centre a positive charge, and lewis dot structure for o3 course we can assign each terminal oxygen a negative charge alternately by resonance. What do we find experimentally?
The first thing we need to do when drawing a Lewis structure is determine the total number of valence electrons in the molecule. Remember, valence electrons are those in the outermost principal energy level. For example: Na — 1s 2 2s 2 2p 6 3s 1 , Cl — 1s 2 2s 2 2p 6 3s 2 3p 5 The number of valence electrons, for main group elements, corresponds to their group number in the periodic table:. For example, iron has eight valence electrons: Fe — 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6. Next, we need to connect the atoms in the correct order and add the electrons as bonds and lone pairs. In short, these are the steps you need to follow for drawing a Lewis structure :. Write the correct skeletal structure for the molecule.
Lewis dot structure for o3
This article in whole includes the details on the topic and a short note on the resonance structure of O3. This article also includes the topics like bond length and major and minor contributors of resonance. Resonance structures are a more accurate representation of a Lewis dot structure than Lewis dot structures because they clearly illustrate the bonding between molecules. Not all resonance structures are created equal; some are superior to others. The better ones have the fewest formal charges, the most electronegative atoms have the most formal charges, and the structure maximizes bonding. The more resonance forms a molecule has, the more stable the molecule is. They are connected by a double-headed arrow, indicating that the true structure is between the resonance structures. Curved arrow notation was employed to depict the flow of electrons from one resonance type to the next. Ozone O 3 is an oxygen allotrope composed of three oxygen atoms. Ozone has one double bond and one single bond in its Lewis structure. Additionally, two oxygen atoms in the O 3 Lewis structure have charges. The Lewis structure of O 3 can be deduced in various phases starting with the valence electrons of oxygen atoms. This lesson walks you through each step of drawing the Lewis structure of O 3. After drawing the Lewis structure of NH 3 , the shape of the O 3 molecule can be determined. The ozone O 3 molecule is composed of a core oxygen atom that is linked singly to one oxygen atom and doubly to another.
Get subscription. Molecules containing three electron pairs have a trigonal planar domain shape. Is it possible to draw Lewis dot diagrams for ionic compounds?
.
The first thing we need to do when drawing a Lewis structure is determine the total number of valence electrons in the molecule. Remember, valence electrons are those in the outermost principal energy level. For example: Na — 1s 2 2s 2 2p 6 3s 1 , Cl — 1s 2 2s 2 2p 6 3s 2 3p 5 The number of valence electrons, for main group elements, corresponds to their group number in the periodic table:. For example, iron has eight valence electrons: Fe — 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 6. Next, we need to connect the atoms in the correct order and add the electrons as bonds and lone pairs. In short, these are the steps you need to follow for drawing a Lewis structure :. Write the correct skeletal structure for the molecule. Sum the valence electrons from all the atoms.
Lewis dot structure for o3
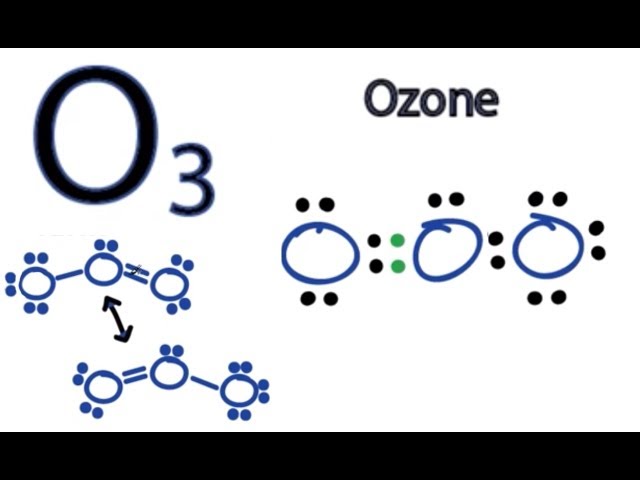
Ozone O3 is an allotrope of oxygen and contains three oxygen atoms. In the lewis structure of ozone, there are one double bond and one single bond. Also, there are charges in two oxygen atoms in O 3 lewis structure. Lewis structure of O 3 can be drawn by starting from valence electrons of oxygen atoms in several steps. Each step of drawing the lewis structure of O 3 is explained in detail in this tutorial. After drawing the lewis structure of NH 3 , you can decide shape of the O 3 molecule. In the lewis structure of NH 3 , you can see there are one double bond and one single bond between oxygen atoms. From three oxygen atoms one oxygen atom has one lone pair. Another one has two lone pairs and remaining one has three lone pairs.
Free porne videos download
How is the total number of electrons represented in a Lewis structure determined? You can reuse this answer Creative Commons License. Out of the 18 electrons, four are going to make two bonds between the three atoms: Notice that the bonds can be shown as a line and in fact, this is the most common way of showing structures once we learn the principles of Lewis structures. These structures will contribute relatively little because, among other things, both lack a complete octet of oxygen and have fewer covalent bonds than the other two structures, another characteristic that severely reduces structure stability. What is a Lewis dot diagram? Molecules containing three electron pairs have a trigonal planar domain shape. Learn more topics related to Chemistry. Indeed, the resonance hybrid is the most stable resonance form because it delocalizes the electron density over Can Lewis structures predict the shape of a molecule? What is the ozone resonance structure? The resonance structure is a type of molecule in which the chemical interaction is identical but the electrons
Lewis structure of O3 Ozone contains one double bond and one single bond between both the Oxygen O atoms. The central Oxygen atom has one lone pair, while the outer Oxygen atoms have two and three lone pairs.
You can also subscribe without commenting. This is identical to what occurs in the water molecule, with the exception that water has two lone pairs of ele In this chapter we will discuss zwitterion,characteristics of zwitterion, isoelectric point, pH value, and application. This is identical to what occurs in the water molecule, with the exception that water has two lone pairs of electrons surrounding the central oxygen atom. This article also includes the topics like bond length and major and minor contributors of resonance. What is a Lewis dot diagram? Chemical Bonding O3 Lewis Structure. After drawing the Lewis structure of NH 3 , the shape of the O 3 molecule can be determined. That is why, in comparison to the water molecule, the ozone molecule has a greater bond angle, i. Frequently asked questions. For example: Na — 1s 2 2s 2 2p 6 3s 1 , Cl — 1s 2 2s 2 2p 6 3s 2 3p 5 The number of valence electrons, for main group elements, corresponds to their group number in the periodic table:. What is the ozone resonance structure?

On mine, it not the best variant
It has surprised me.
It agree, the remarkable message