Lewis dot structure for cocl2
By now, you have probably noticed a pattern in covalent bond formation. When atoms form the normal number of covalent bonds with other elements, they may do so in any manner that sums to equal the normal number of bonds.
Name the third and fourth transition elements of first transition series. What is the coordinate number of the central metal ions in the following coordination compound? The resistance of a 0. Calculate the molar conductivity of the solution if the electrods in the cell are 1. Draw the Lewis dot structures for sulphurtrioxide.
Lewis dot structure for cocl2
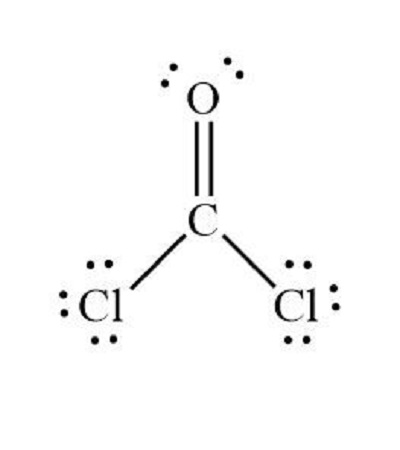
When determining the formal charge of a molecule such as CoCl2 phosgene gas , you need to know the number of valence electrons for each atom and the Lewis structure of the molecule. Look up each atom in the periodic table of elements to determine the number of valence electrons. Remember that two electrons go in the first s shell, two electrons in the second s shell, six electrons in the first p shell, etc. Adjust for charge. If the molecule is an ion, add or subtract one or more electrons overall to account for the final charge. The molecule is not ionized and has a neutral charge. See the diagram for the Lewis structure of CoCl2 phosgene gas. The Lewis structure represents the most stable and probable structure for a molecule. Atoms are drawn with paired valence electrons; bonds are formed between lone electrons to satisfy the octet rule. The chloride atoms share single bonds with the carbon molecule, while the oxygen atom forms a double bond with carbon. Each atom in the final structure satisfies the octet rule and has eight valence electrons allowing for molecular stability. Count the lone pairs of each atom in the Lewis structure. Assign each atom one electron from each bond in which it participates. Add these numbers together.
How to Calculate a Steric Number.
Carbon, in group 4 or 14, has 4 valence electrons. Oxygen in group 6, sometimes called 16, 6 valence electrons. Seven for Chlorine but we have two Chlorines. Add it all up, 4 plus 6 plus 14, you have a total of 24 valence electrons. Carbon is the least electronegative.
Phosgene is a colorless gaseous compound known as carbonyl chloride and has a molecular weight of It is non-flammable in nature and bears a suffocating odor. It has a boiling point b. Phosgene is acyl chloride. It is used to manufacture precursors for herbicide production and used to manufacture pharmaceuticals and pesticides. Other than this, COCl2 is needed to produce certain polycarbonate compounds which in turn are utilized for plastic production in eye lenses and other appliances. It is highly poisonous and toxic in nature and therefore needs to be handled with caution and via safety precautions.
Lewis dot structure for cocl2
COCl 2 phosgene has one carbon atom, one oxygen atom, and two chlorine atoms. In the COCl 2 Lewis structure, there are two single bonds and one double bond around the carbon atom, with two chlorine atoms and one oxygen atom attached to it. Two chlorine atoms with single bonds have three lone pairs, and one oxygen atom with a single bond has two lone pairs.
Bros lecce
How to Calculate Bond Energy. Final check: The total number of electrons shown in the molecule must equal the number of valence electrons calculated in the first step. N is the central atom since it makes the most bonds. Also, the molecule is linear, since the central C atom is only bonded to two other atoms and there are no lone pairs on the C. How to Calculate Valency of Radicals. Put the Oxygen and then the two Chlorines around the outside. A molecule with a few atoms is more likely to be build symmetrically around a central atom rather than in a straight line. Both C and N are following the octet rule. The chloride atoms share single bonds with the carbon molecule, while the oxygen atom forms a double bond with carbon. How to Tell if a Molecule Is Bent. But in the center, Carbon only has 6 valence electrons. So this is the Lewis structure for COCl2. An N atom makes 3 bonds, while an F atom makes 1 bond. How many grams of potassium dichromate are required to oxidise
Carbon, in group 4 or 14, has 4 valence electrons.
So by sharing these two valence electrons up here with the Carbon, and forming a double bond, we now have used 24 valence electrons and each of the atoms has an octet. The atomic number of cerium Ce is Choose the wrong statement in the following: Construct a skeleton structure for the molecule. When determining the formal charge of a molecule such as CoCl2 phosgene gas , you need to know the number of valence electrons for each atom and the Lewis structure of the molecule. Calculate the molar conductivity of the solution if the electrods in the cell are 1. Draw the Lewis dot structures for sulphurtrioxide. N is the central atom since it makes the most bonds. What is the coordinate number of the central metal ions in the following coordination compound? Normal number of covalent bonds:.

Did not hear such
I regret, that I can help nothing. I hope, you will find the correct decision. Do not despair.
As well as possible!