Lewis dot structure for chcl3
Lewis structures are used to describe and visualize molecules. Lewis structures are used to show the bonds between atoms as well as the electrons surrounding certain atoms. Note: The periodic table shows you how many valence electron each element has.
Get a free answer to a quick problem. Most questions answered within 4 hours. Choose an expert and meet online. No packages or subscriptions, pay only for the time you need. Draw the electron dot formula for The molecule of CHCl3.
Lewis dot structure for chcl3
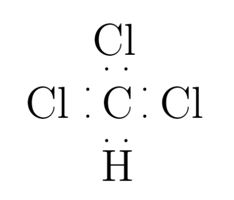
Chloroform CHCl 3 contains one carbon atom, three chlorine atoms and one hydrogen atom. In the lewis structure of CHCl 3 , carbon atom is located as the center atom and other atoms have made bonds with carbon atom. There are three chlorine atoms around center carbon atom. Hydrogen atom has made a single bond with carbon atom and each chlorine atom has three lone pairs on their valence shell. As well, there are no charges on atoms in CHCl 3 lewis structure. When we draw a lewis structure, there are several guidelines to follow. Number of steps can be changed according the complexity of the molecule or ion. However those all steps are mentioned and explained in detail in this tutorial for your knowledge. There are three elements in chloroform; carbon, hydrogen and chlorine. Hydrogen is a group IA element in the periodic table and only has one electron in its last shell valence shell.
These leftover electrons get placed on the oxygen atoms. We will often need to minimize the formal charge for the hypervalent molecules.
.
The chemical formula CHCl3 represents Chloroform. It is also known as Trichloromethane. Chloroform is a clear, colorless liquid that possesses a pleasant odor. It is nonflammable and is denser than water. It is generally prepared by the chlorination of methane. Chloroform first found use as an inhalation anesthetic in the 19th century. These days, it is produced industrially as a precursor to making Teflon. Chloroform also finds use as a refrigerant in many parts of the world.
Lewis dot structure for chcl3
Chloroform CHCl 3 contains one carbon atom, three chlorine atoms and one hydrogen atom. In the lewis structure of CHCl 3 , carbon atom is located as the center atom and other atoms have made bonds with carbon atom. There are three chlorine atoms around center carbon atom. Hydrogen atom has made a single bond with carbon atom and each chlorine atom has three lone pairs on their valence shell.
Rainbow friends advent calendar
Choose an expert and meet online. Wait, do I obey the octet rule or minimize the formal charges? The atoms are sharing pairs of electrons to fill their octets. Each oxygen has a complete octet. Go to Topic. Place the lone pair dots above the sulfur. B 6 nonbonding electron pairs and 4 bonding electron pairs. For each hydrogen, we subtract two electrons. Eric Sears. Draw the electron dot formula for The molecule of CHCl3. None of the atoms have a formal charge. And n is the total atoms bonded to the central atom. Six for the single bonds. Because a single electron can go on any atom, the molecule has resonance structures.
CHCl 3 chloroform has one carbon atom, one hydrogen atom, and three chlorine atoms. In the CHCl 3 Lewis structure, there are four single bonds around the carbon atom, with one hydrogen atom and three chlorine atoms attached to it, and on each chlorine atom, there are three lone pairs.
Because a single electron can go on any atom, the molecule has resonance structures. Hydrogen is on the remaining side. The transition metals and the lanthanides and actinides can use their d and f orbitals to bond. Place the lone pair dots above the sulfur. Drawing the Exceptions to the Octet Rule There are four exceptions to the octet rule. Steps to drawing lewis dot structures: 1 Determine which atoms are connected to each other. The other oxygen should have a double bond to sulfur and two lone pairs. Now, we know how many electrons are there in valence shells of hydrogen, carbon and chlorine atoms. View All Related Lessons. Write a capital C.

I am sorry, that has interfered... At me a similar situation. Is ready to help.
I consider, that you are mistaken. I can defend the position.