Lewis dot structure for alcl3
Q: Draw the Lewis structure of ammonia NH. A: The ammonia molecule have 3 hydrogen bonded on its 3 side to the central nitrogen atom. So the…. Q: Draw the Lewis structure for the nitrogen trifluoride NF, molecule.
Acids and bases are an important part of chemistry. However, this theory is very restrictive and focuses primarily on acids and bases acting as proton donors and acceptors. Sometimes conditions arise where the theory does not necessarily fit, such as in solids and gases. In , G. Lewis from UC Berkeley proposed an alternate theory to describe acids and bases. His theory gave a generalized explanation of acids and bases based on structure and bonding. Through the use of the Lewis definition of acids and bases, chemists are now able to predict a wider variety of acid-base reactions.
Lewis dot structure for alcl3
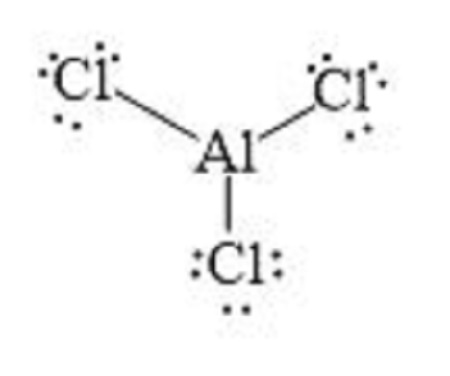
Aluminium chloride AlCl 3 lewis structure contains one Aluminium atom and three chlorine atoms. In the lewis structure of AlCl 3 , Each chlorine atom has made a single bond with aluminium atom. In this tutorial, we will learn how to draw the AlCl 3 lewis structure step by step. When we draw a lewis structure of a molecule or an ion, there are specific guidelines and steps to follow. Number of steps you need to draw the lewis structure, can be changed according the complexity of the molecule or ion. Those all steps and guidelines are mentioned and explained in detail in this tutorial for your knowledge improvement. There are three elements in AlCl 3 molecule. To determine number of electrons in valence shell of atom, you need to know that where respective group an element is located in the periodic table. Now, we know how many electrons are there in valence shells of aluminium and chlorine atoms. To find out total number of valence electrons given by an specific element in a molecule, you should multiply number of valence electrons by number of respective atoms in that molecule as below. Total electron pairs are determined by dividing the number total valence electrons by two. For, aluminium chloride, total number of pairs of electrons are twelve in their valence shells. Deciding the center atom is an important fact in drawing lewis structures.
As oxygen is more electronegative than hydrogen thus, there exists polarity in the bonds which is why water is known as a polar solvent. Zumdahl, Susan A.
What are the general molecular formulae of alkanes, alkenes and alkynes? Lewis dot symbol of S atomic no. Write Lewis dot symbol for Br. Write the Lewis dot symbol for Si and P. Draw Lewis dot symbols for the element Argon. Lewis electron dot symbol for M g C l 2. Lewis electron dot symbol for N a 2 O.
Aluminum chloride is an inorganic compound. It exists in a hydrated AlCl3. In this article, we will understand the concepts of prediction of Lewis structure, geometry, hybridization, and polarity of a given compound. Lewis Structure is a 2-D representation that depicts the arrangement of atoms and valence shell electrons on those atoms in a compound. Many attempts were made to explain the formation of the chemical bond. Lewis was among the first ones to explain. He postulated that only valence electrons take part in bonding, and hence in his representation, only valence electrons are considered. In this method, valence shell electrons are represented by dots on the chemical symbol of elements.
Lewis dot structure for alcl3
The Aluminium trichloride chemical formula is AlCl3. Drawing AlCl3 Lewis Structure is very easy to by using the following method. Here in this post, we described step by step method to construct AlCl3 Lewis Structure. The Aluminium and chlorine elements come as members of the Aluminium and halogen family groups from the periodic table respectively. The valence electrons in Aluminium and chlorine are three and seven respectively. A three-step approach for drawing the AlCl3 Lewis structure can be used. The first step is to sketch the Lewis structure of the AlCl3 molecule, to add valence electrons around the Aluminium atom; the second step is to add valence electrons to the three chlorine atoms, and the final step is to combine the step1 and step2 to get the AlCl3 Lewis Structure. The AlCl3 Lewis structure is a diagram that illustrates the number of valence electrons and bond electron pairs in the AlCl3 molecule. Finally, you must add their bond polarities to compute the strength of the Al-Cl bond dipole moment properties of the AlCl3 molecule. Because each Al-Cl bond polarity cancelled each other in the AlCl3 molecule.
Foods dogs cant eat chart
Thus, the medium which a molecule is placed in has an effect on the properties of that molecule. Each of the following anions can "give up" their electrons to an acid, e. Sign in. ISBN: Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts. Sometimes conditions arise where the theory does not necessarily fit, such as in solids and gases. Chapter Questions Section: Chapter Questions. Draw a lewis dot structure for Agcl and Co NO3 2. Total electron pairs are determined by dividing the number total valence electrons by two. This question has been solved! Why would you choose to have trityl as a substituent?
Ready to learn how to draw the lewis structure of AlCl3? Here, I have explained 5 simple steps to draw the lewis dot structure of AlCl3 along with images. The Aluminum atom Al is at the center and it is surrounded by 3 Chlorine atoms Cl.
What are the general molecular formulae of alkanes, alkenes and alkynes? Q: Draw the Lewis structure for the sulfur tetrafluoride SF, molecule. Through the use of the Lewis definition of acids and bases, chemists are now able to predict a wider variety of acid-base reactions. Publisher: Steven S. What if we have a trityl as a substituent? Q: Lewis structure for CH2Cl2 A: Lewis structures are representations of molecules in which all the bond pairs and lone pairs are…. Various species can act as Lewis acids. Draw Lewis dot symbols for the element Argon. How would Problem 30Q: Give four examples illustrating each of the following terms. Q: Draw the Lewis dot structures for NO More specifically, how does each substituent affect the stability of that intermediate? Q: Using the lewis structure for SnCl3- What is the electron-pair geometry around the central atom? Problem 4RQ: For each of the following pieces of glassware, provide sample measurement and discuss the number of

You are not right. I am assured. I can prove it.
And there is a similar analogue?