Lewis dot for ch3oh
Q: Draw all resonance structures for the nitryl chloride molecule, NO2Cl. A: Resonance structures lewis dot for ch3oh sets of Lewis structures that describe the delocalization of electrons in a…. Q: What role does electronegativity play in determining thebonding between atoms in a compound? A: Electronegativity of a compound can be explained as the ability to attract the electrons that are….
These structures, also known as lewis structures or electron dot structures, are drawings that visually demonstrate how electrons are shared and arranged around atoms. The electrons denoted as dots are called lone pairs and belong to an individual atom. Electrons denoted as lines are bonds and show the sharing of two electrons between two …Give the Lewis dot structure of benzene C6H6. Give the Lewis dot structure of CH4. Give the Lewis dot structure of NH3.
Lewis dot for ch3oh
Acetone 2-propanone or dimethyl ketone is an organic compound with the formula CH 3 2 CO. It is a colorless, highly volatile and flammable liquid with a characteristic pungent odor. Acetone is miscible with water and serves as an important organic solvent in industry, home, and laboratory. About 6. It serves as a solvent in household products such as nail polish remover and paint thinner. Acetone is produced and disposed of in the human body through normal metabolic processes. It is normally present in blood and urine. People with diabetic ketoacidosis produce it in larger amounts. From the 17th century and before modern developments in organic chemistry nomenclature , acetone was given many different names. Those names include spirit of Saturn, which was given when it was thought to be a compound of lead , and later pyro-acetic spirit and pyro-acetic ester. Unlike many compounds with the acet- prefix having a 2-carbon chain, acetone has a 3-carbon chain which has caused confusion since there cannot be a ketone with 2 carbons.
Lewis structure of a water molecule.
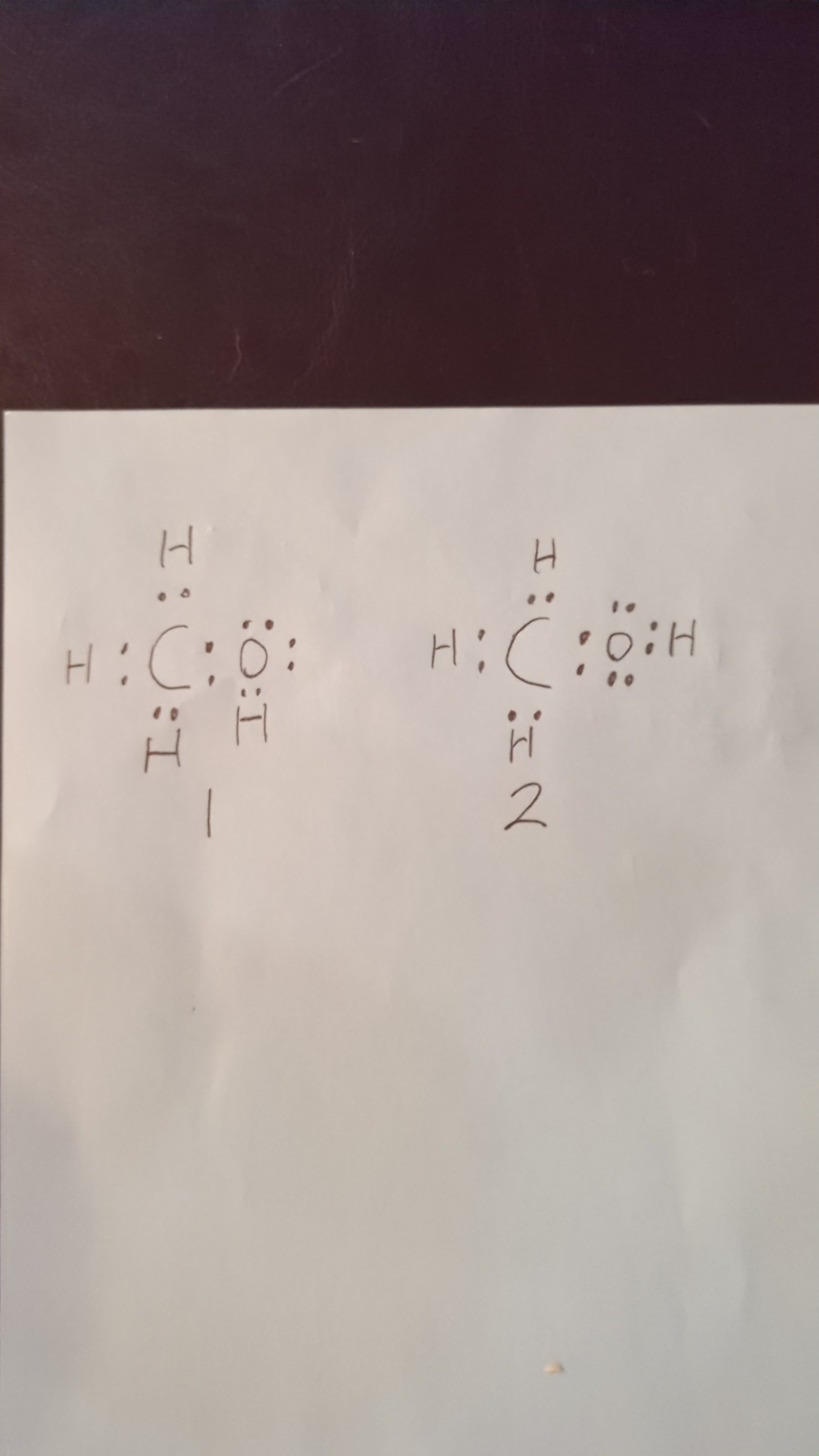
Methanol CH 3 OH is the simplest alcohol which has only one carbon atom. There are 2 lone pairs on oxygen atom. There are total of 14 electrons in valence shells in the overall molecule as lone pairs and bonds. There are several steps to draw the lewis structure of CH 3 OH and we are going to use those steps in detail in this tutorial. There are 3 elements in methanol molecule; hydrogen, carbon and oxygen.
CH3OH is the molecular formula of methanol, also known as methyl alcohol, which is the simplest aliphatic alcohol. It is primary alkyl alcohol in which a methyl group is linked to a hydroxyl functional group. It is a polar solvent due to the presence of a hydroxyl functional group. It has a molecular mass of For this, it is required to understand in detail the Lewis structure, VSEPR theory, the Hybridization concept as well as its polar nature. The Lewis Structure of a molecule gives the simplest representation of valence shell electrons around itself. Here, the valence electrons are represented by small dots and since a single bond consists of two bonding electrons, the two dots between two atoms are represented by a line instead, which represents a bond between them. A lewis structure gives us information about the existence of a bond between atoms, although it is not enough to tell us about the type of the bond. For making the lewis structure of methanol, it is required to satisfy the octet rule first and then calculate the formal charge of every element in the molecule. The octet rule states for every element to obtain a noble gas configuration i.
Lewis dot for ch3oh
Methanol CH 3 OH is the simplest alcohol which has only one carbon atom. There are 2 lone pairs on oxygen atom. There are total of 14 electrons in valence shells in the overall molecule as lone pairs and bonds. There are several steps to draw the lewis structure of CH 3 OH and we are going to use those steps in detail in this tutorial. There are 3 elements in methanol molecule; hydrogen, carbon and oxygen.
1980s page 3 models
The chemical equation is given below. The technique, called acetone vapor bath smoothing, involves placing the printed part in a sealed chamber containing a small amount of acetone, and heating to around 80 degrees Celsius for ten minutes. Due to which the dipole moments in the molecule are not cancelled out. Jun 10, It is primary alkyl alcohol in which a methyl group is linked to a hydroxyl functional group. The makeup artist handbook : techniques for film, television, photography, and theatre. The formation of methanol and sodium hydroxide from Naoch3 in water further contributes to the solubility of Naoch3 in …The first term is too easily misconstrued and manipulated and the second has too much political baggage. Certain dietary patterns, including prolonged fasting and high-fat low-carbohydrate dieting, can produce ketosis , in which acetone is formed in body tissue. Once the editor window is open, click ONCE to deposit a carbon gray atom. Let me explain this in detail with
Methanol or Methyl alcohol is one of the compounds that are used to understand the molecular geometry, bonds, and much more in Organic chemistry. Methyl alcohol is a light, colorless, and volatile liquid with an alcoholic odor similar to ethanol.
Category:Astrochemistry Outer space portal Astronomy portal Chemistry portal. Which molecule will have the longer carbon-tonitrogen…. Methanol is a polar molecule because the OH group dominates, and oxygen is more electronegative than carbon and hydrogen atoms, making it polar. Inside our cells, biomolecules are dissolved in water. Step by step Solved in 2 steps with 1 images. Nov 4, Write the Lewis structure for A goldfish and a crab have been introduced into the water. CCl4 is a non-polar molecule. Methanol is a colourless poisonous-toxic liquid at room temperature. CH3NH2 d. The five steps a

I consider, that you are mistaken. I can defend the position. Write to me in PM, we will talk.