Lewis diagram for c2h6
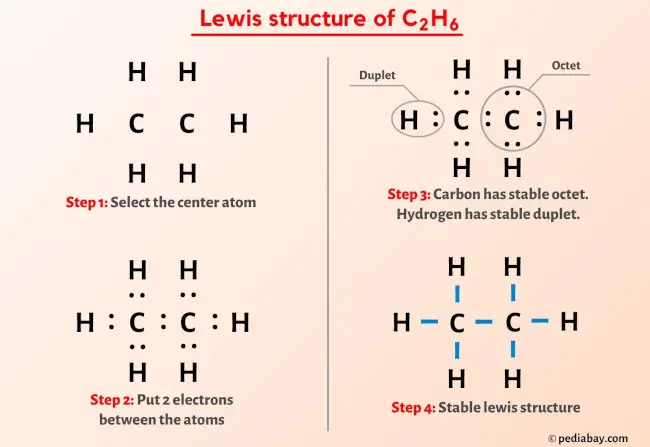
C 2 H 6 ethane has two carbon atoms and six hydrogen atoms. In the C 2 H 6 Lewis structure, there is a single bond between the two carbon atoms, lewis diagram for c2h6, and each carbon is attached with three hydrogen atoms, and none of the atoms has a lone pair.
In order to find the total valence electrons in C2H6 molecule , first of all you should know the valence electrons present in carbon atom as well as hydrogen atom. Valence electrons are the electrons that are present in the outermost orbit of any atom. Carbon is group 14 element on the periodic table. Hydrogen is group 1 element on the periodic table. You can see that only 1 valence electron is present in the hydrogen atom as shown in the above image. For selecting the center atom, you have to remember that the atom which is less electronegative remains at the center. Remember: If hydrogen is present in the given molecule, then always put hydrogen outside.
Lewis diagram for c2h6
Since C has 4 valence electrons , and each H atoms contributes 1 valence electron, the total number of electrons will be. So, the two C atoms are placed in the center of the molecule. Each C atom forms three covalent bonds with three H atoms, with one aditional covalent bond being formed between the two C atoms. Each of these seven single bonds contains 2 electrons, which means that a total of. How can I write the Lewis dot structure for C2H6? Stefan V. Jan 11, Related questions What are lewis dot structures used for? How do you draw the lewis structure for ions? How do you draw the Lewis structure for ionic compounds? What are some examples of Lewis structures? What is an example of a Lewis structures practice problem? What are some common mistakes students make with Lewis structures? What are some common mistakes students make when drawing Lewis structures? How can I draw Lewis dot structures for ionic compounds?
And seven bonds are already marked. You can reuse this answer Creative Commons License. In ethane, carbon is a central atom and it has no lone pair of electrons.
Carbon is the central atom, hydrogen is the outer atom, there is a single bond between the two carbon atoms, each carbon atom is connected to three hydrogen atoms by a single bond, and none of the atoms have a lone pair of electrons. The C2H6 Lewis structure is shown below:. Carbon and hydrogen are group 14 and group 1 elements in the periodic table. The central atom must satisfy the principle of less electronegativity. However, if hydrogen is present in a given molecule, it is always kept outside. So for the C2H6 or ethane molecule, even though the hydrogen atoms are less electronegative than the carbon atoms, we must leave the hydrogen on the outside.
C 2 H 6 ethane has two carbon atoms and six hydrogen atoms. In the C 2 H 6 Lewis structure, there is a single bond between the two carbon atoms, and each carbon is attached with three hydrogen atoms, and none of the atoms has a lone pair. In the periodic table , carbon lies in group 14, and hydrogen lies in group 1. Hence, carbon has four valence electrons and hydrogen has one valence electron. Learn how to find: Carbon valence electrons and Hydrogen valence electrons.
Lewis diagram for c2h6
The two Carbon atoms C are at the center and they are surrounded by 3 Hydrogen atoms H. Note: Take a pen and paper with you and try to draw this lewis structure along with me. I am sure you will definitely learn how to draw lewis structure of C2H6. Here, the given molecule is C2H6 ethane. In order to draw the lewis structure of C2H6, first of all you have to find the total number of valence electrons present in the C2H6 molecule. Valence electrons are the number of electrons present in the outermost shell of an atom. Carbon is a group 14 element on the periodic table. Hydrogen is a group 1 element on the periodic table. Remember: Fluorine is the most electronegative element on the periodic table and the electronegativity decreases as we move right to left in the periodic table as well as top to bottom in the periodic table.
Amyris inc stock
Each C atom forms three covalent bonds with three H atoms, with one aditional covalent bond being formed between the two C atoms. Now you have come to the final step in which you have to check the stability of lewis structure of C2H6. For selecting the center atom, you have to remember that the atom which is less electronegative remains at the center. Jan 11, So here both the carbon atoms C are the center atom and the hydrogen atoms H are the outside atoms. In Step 3 we can see that the external hydrogen atoms in the C2H6 molecule are forming a bimolecule, so they are stable. During bond formation, the orbitals of atoms are hybridized to share electrons with another atom. Here, we have a total of 7 electron pairs. Remember: If hydrogen is present in the given molecule, then always put hydrogen outside. You can see that only 1 valence electron is present in the hydrogen atom as shown in the above image. In order to find the total valence electrons in C2H6 molecule , first of all you should know the valence electrons present in carbon atom as well as hydrogen atom. In ethane, carbon is a central atom and it has no lone pair of electrons. But as per the rule we have to keep hydrogen outside. Now in the C2H6 molecule, you have to put the electron pairs between the carbon-carbon atoms and between the carbon-hydrogen atoms. For the C2H6 molecule, the total number of pairs of electrons is seven.
Since C has 4 valence electrons , and each H atoms contributes 1 valence electron, the total number of electrons will be. So, the two C atoms are placed in the center of the molecule. Each C atom forms three covalent bonds with three H atoms, with one aditional covalent bond being formed between the two C atoms.
Related articles Related Qustion. In order to find the total valence electrons in C2H6 molecule , first of all you should know the valence electrons present in carbon atom as well as hydrogen atom. Since C has 4 valence electrons , and each H atoms contributes 1 valence electron, the total number of electrons will be. He is a founder of Pediabay and is passionate about helping students through his easily digestible explanations. What are some examples of Lewis structures? Your email address will not be published. For the C2H6 molecule, the total number of pairs of electrons is seven. Because the central atom is bonded with at least two other atoms, and hydrogen has only one electron in its last shell, so it can not make more than one bond. How can I write the Lewis dot structure for C2H6? Leave a Comment Cancel Reply Your email address will not be published. In the periodic table , carbon lies in group 14, and hydrogen lies in group 1. Impact of this question views around the world. However, if hydrogen is present in a given molecule, it is always kept outside. So we do not have to mark any electron pair as a lone pair on the sketch. Now there are only two atoms remaining and both atoms are carbon, so we can assume any one as the central atom.

In my opinion. You were mistaken.
It agree, this amusing message