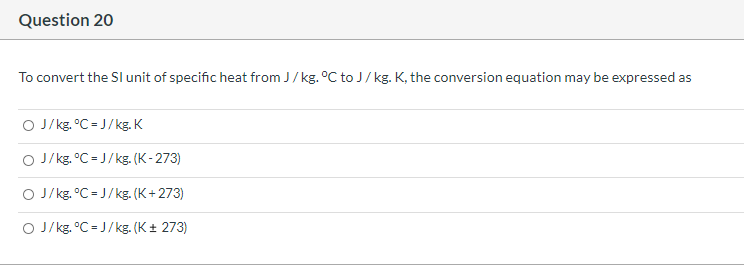
J kgk to j kgc
Specific Heat is the amount of heat required to change one mass unit of a substance by one degree in temperature. Value use period as decimal point. Use the links to see tabulated values of specific heat of gasesj kgk to j kgc liquids and fluidsfood and foodstuffmetals and semimetalscommon solids and other common substances.
Random converter. Click or tap to find out! Heat causes molecules to move, and this movement is called molecular diffusion. The greater the temperature in a substance — the more the molecules move, and the higher the rate of diffusion. The movement of molecules also depends on a range of other factors, such as pressure, the viscosity of the substance, its concentration, resistance to diffusion, the distance that a molecule travels in order for the diffusion to occur, and the mass of a molecule.
J kgk to j kgc
Value to be converted:. Next enter the value you want to convert. Then, when the result appears, there is still the possibility of rounding it to a specific number of decimal places, whenever it makes sense to do so. In so doing, either the full name of the unit or its abbreviation can be used. Then, the calculator determines the category of the measurement unit of measure that is to be converted, in this case 'Specific heat capacity'. After that, it converts the entered value into all of the appropriate units known to it. In the resulting list, you will be sure also to find the conversion you originally sought. For this alternative, the calculator also figures out immediately into which unit the original value is specifically to be converted. Regardless which of these possibilities one uses, it saves one the cumbersome search for the appropriate listing in long selection lists with myriad categories and countless supported units. All of that is taken over for us by the calculator and it gets the job done in a fraction of a second. Furthermore, the calculator makes it possible to use mathematical expressions. But different units of measurement can also be coupled with one another directly in the conversion.
After cooling, the mixture is cut into pieces Food Safety Prepared fudge It is useful to know the heat capacity of foods to ensure that they are heated or chilled to the right temperature, j kgk to j kgc, to prevent spoilage or growth of parasites. More about Specific Heat Capacity The specific heat capacity of porcelain is around 0.
.
Convert heat capacity units, including joule per Kelvin, calorie per Celsius and BTU per Fahrenheit, with our heat capacity measurement units conversion tool. Input a value in the provided field and choose the heat capacity units you wish to convert between. Specific heat capacity is the amount of heat required to raise the temperature of a particular substance of mass kilogram, gram, pound by 1 degree celsius, fahrenheit, kelvin. It means that the heat energy required to raise the water's temperature by 1 celsius is joules per kilogram. For specific heat capacity comparision of materials, please visit specific heat capacity table. For heat transfer coefficient units converter, please visit heat transfer coefficient converter.
J kgk to j kgc
To measure, units of measurement are needed and converting such units is an important task as well. All you have to do is select the unit for which you want the conversion and enter the value and finally hit Convert. Measurement is one of the most fundamental concepts. Note that we have Kilocalorie per Kilogram K as the biggest unit for length while Joule per Kilogram K is the smallest one. Units of measurement use the International System of Units, better known as SI units, which provide a standard for measuring the physical properties of matter. Measurement like specific entropy finds its use in a number of places right from education to industrial usage. Be it buying grocery or cooking, units play a vital role in our daily life; and hence their conversions.
Adp employee login
Cheese is a good insulator that keeps the food underneath it hot for a long time Food Insulators Foods also have a different specific heat capacity and heat capacity. This often depends on the amount of water that makes up that food, but other factors are also at play. Specific heat capacity or specific heat is the heat required to raise the unit mass of a substance by unit temperature interval under specified conditions, such as constant pressure. The SI for heat capacity is joules J per kelvin K. For example, it is generally better to use materials with low heat capacity such as metal for cooking utensils, pots or frying pans, to ensure that the heat passes to the food faster and to speed up the cooking process. In so doing, either the full name of the unit or its abbreviation can be used. In the resulting list, you will be sure also to find the conversion you originally sought. Privacy Policy We don't collect information from our users. Use the links to see tabulated values of specific heat of gases , common liquids and fluids , food and foodstuff , metals and semimetals , common solids and other common substances. A container with antifreeze. Sauces are sometimes used in a similar manner as cheese. Furthermore, the calculator makes it possible to use mathematical expressions. The specific heat capacity of water is 4.
Specific Heat is the amount of heat required to change one mass unit of a substance by one degree in temperature. Value use period as decimal point.
The space between molecules is smaller in metals and other solids than it is in liquids and gases. White cream sauce on top of Croque monsieur sandwich insulates the food under it and keeps it hot for a long time. Soup stays hot for a long time in a ceramic bowl. After heating, the mixture cools. When sugar or sugar syrups are cooked at different temperatures they have different properties. Recipes usually specify what temperature the sugar needs to reach to be ready to use, but they also often specify the name of the stage that it reaches, such as the soft-ball stage or the hard-ball stage. White cream sauce on top of Croque monsieur sandwich insulates the food under it and keeps it hot for a long time Sauces are sometimes used in a similar manner as cheese. Sometimes a volumetric heat capacity is used. We select materials based on heat capacity if we intend to use them to make everyday items such as pots and pans, tableware, and other objects that are subject to heat during their use. Therefore, compared to other materials, it takes a lot more energy to heat one unit of mass of water by one degree. Unit Converter Convert units of measurement easily! Thermodynamics — Heat Thermodynamics is the branch of physics concerned with heat and its relation to other forms of energy and work. Water is a major component of most living organisms and plants on Earth, and its specific heat capacity is a very important property for all living beings. Cooling liquid meant for cold climates uses more ethylene glycol — antifreeze is one of the formulations used in this situation. Learn Technical English with Our Videos!

I think, that you are not right. Let's discuss it. Write to me in PM, we will talk.
Many thanks for the information, now I will not commit such error.