Is so2 polar
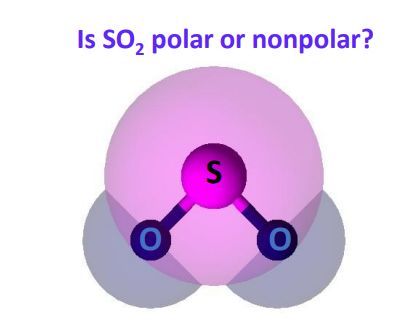
To determine if SO 2 sulfur dioxide is polar or nonpolar, we need to first determine its geometry. This presumes knowing the rules for drawing a correct Lewis structure is so2 polar you can find more details about Lewis structures here. The two oxygens take 6 lone pairs, and the remaining one goes to the sulfur:.
For instance, water is a polar molecule while carbon dioxide is a nonpolar molecule. What about sulfur dioxide , is it polar or nonpolar? Sulfur dioxide is considered a polar molecule. What exactly does being a poor molecule mean? Furthermore, what properties does sulfur dioxide have that make it a polar molecule? These are the top and bottom areas of the earth.
Is so2 polar
.
Check this question multiple-choice quiz on Geometry and Hybridization: Is so2 polar. Therefore, one lone pair from each oxygen is used to make an additional bond with the sulfur: The central atom has a steric number of 3 — two atoms and one lone pair. The two oxygens take 6 lone pairs, and the remaining one goes to the sulfur: As it is drawn, the problems with this structure are that the sulfur lacks an octet and the oxygens have only one bond and three lone pairs, is so2 polar.
.
Hello friends, you might have many doubts regarding the polarity in some molecules in the chemistry world. Many of us have a doubt regarding the polarity of SO2 sulfur dioxide. So, I will share my information with you to clear the doubt regarding the polarity of SO2. Is SO2 polar or nonpolar? SO2 is polar in nature because of the difference in electronegativity between sulfur and oxygen atoms.
Is so2 polar
The chemical formula SO 2 represents the chemical compound Sulfur Dioxide. The substance is a colorless gas with a recognizable pungent odor similar to the smell of a burnt matchstick. A large quantity of SO 2 is released during volcanic eruptions. It is also found in some hot water springs.
Security guard hiring now
A difference of 1. Thus the molecule maintains its balance as a nonpolar molecule. Ionic bonds are formed between metals and nonmetals. The electron geometry, therefore, is trigonal planar , and the molecular geometry is bent. You can also subscribe without commenting. This means the oxygen is exerting more pull on the covalent bonds in sulfur dioxide. This means that water is a polar molecule. What about sulfur dioxide , is it polar or nonpolar? These are the top and bottom areas of the earth. By Daniel Nelson.
To determine if SO 2 sulfur dioxide is polar or nonpolar, we need to first determine its geometry. This presumes knowing the rules for drawing a correct Lewis structure and you can find more details about Lewis structures here. The two oxygens take 6 lone pairs, and the remaining one goes to the sulfur:.
When trying to determine the polarity of a molecule, you can use a three-step process to analyze it. Water is one of the most famous polar molecules, and its structure is responsible for making the molecule have a polar nature. They are the ends of the molecules that have either a negative charge or positive charge, much like a battery has a negative end and a positive end. In the case of sulfur dioxide, the molecule is angled and possesses a difference in electronegativity with the pull of sulfur being less than that of oxygen. The electrons within molecules are constantly being pulled around. What about sulfur dioxide , is it polar or nonpolar? However, when there are two atoms of the same type that make up a bond, the electrons within the bond will shift position because the amount of pull that each atom has is equivalent and the electrons that each atom possesses will stay where they are. As an example of a molecule with more negative bonds that is nonpolar, look at carbon dioxide. Sulfur dioxide is considered a polar molecule. One of the reasons that ethane is a nonpolar molecule is that the molecule has a symmetrical structure. To determine if SO 2 sulfur dioxide is polar or nonpolar, we need to first determine its geometry.

And indefinitely it is not far :)