Is so2 polar or nonpolar
Wiki User. The fact that they are joined by polar covalent bonds is irrelevant as interpals penpals bonds do not usually determine the polarity of intramolecular bonds. Sulphur dioxide is angular in shape, presumably due to the extra electron shell as sulphur and oxygen are in the same group. This means one side of the molecule is more negative than the other and vise versa, is so2 polar or nonpolar.
For instance, water is a polar molecule while carbon dioxide is a nonpolar molecule. What about sulfur dioxide , is it polar or nonpolar? Sulfur dioxide is considered a polar molecule. What exactly does being a poor molecule mean? Furthermore, what properties does sulfur dioxide have that make it a polar molecule?
Is so2 polar or nonpolar
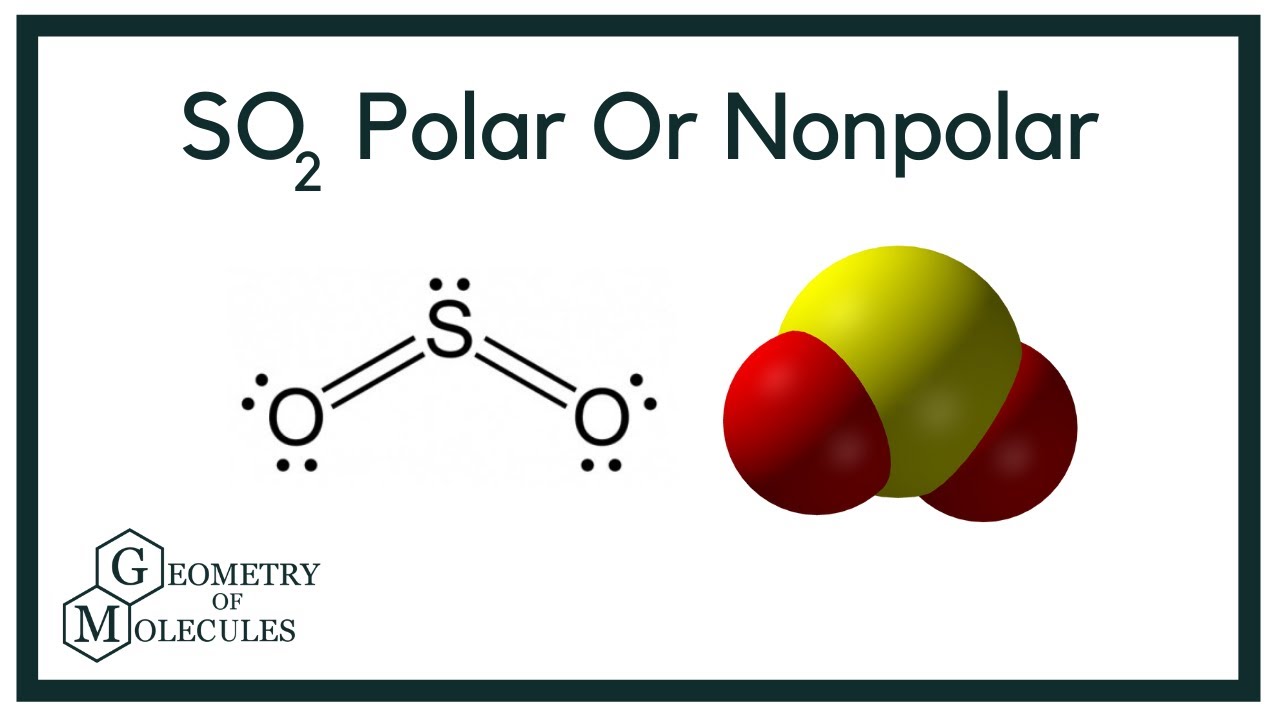
To determine if SO 2 sulfur dioxide is polar or nonpolar, we need to first determine its geometry. This presumes knowing the rules for drawing a correct Lewis structure and you can find more details about Lewis structures here. The two oxygens take 6 lone pairs, and the remaining one goes to the sulfur:. As it is drawn, the problems with this structure are that the sulfur lacks an octet and the oxygens have only one bond and three lone pairs. Remember, the normal valency of oxygens is having two bonds and two lone pairs otherwise a formal charge needs to be assigned. The central atom has a steric number of 3 — two atoms and one lone pair. The electron geometry, therefore, is trigonal planar , and the molecular geometry is bent. Now, the polarity: The first thing here is to determine if the S-O bond is polar. Depending on the difference in the electronegativity values, covalent bonds can be polar and nonpolar. Oxygen is more electronegative and because the dipoles of S-O bonds do not cancel, the molecule is polar. Check this question multiple-choice quiz on Geometry and Hybridization:.
What about sulfur dioxideis it polar or nonpolar? All Rights Reserved. Much like H2O, sulfur is found in the middle of the molecule, with bent bonds connecting the sulfur to the oxygen.
.
The chemical formula SO 2 represents the chemical compound Sulfur Dioxide. The substance is a colorless gas with a recognizable pungent odor similar to the smell of a burnt matchstick. A large quantity of SO 2 is released during volcanic eruptions. It is also found in some hot water springs. Sulfur Dioxide contributes to global warming as a proponent of the greenhouse effect. Sulfur Dioxide is manufactured on an industrial scale by burning or roasting Sulfur and its components Sulfide ores, Sulfites in the presence of Oxygen. The gas released is captured and primarily used in the production of Sulfuric Acid through the contact process.
Is so2 polar or nonpolar
We learned in Section However, when two nonmetals come together, they will share electrons with each other to form covalent bonds as we learned in Section When describing a covalent bond, the implication was that the two electrons in the bond were shared equally between the two nuclei involved.
Yugioh zexal episode guide
What influences how electrons are shifted around is the bonds that exist between molecules. But it has polar covalent bonds between its atoms. The atom that has the greater ability to pull electrons towards itself will have an increased number of electrons around it, it will have a slightly more negative charge overall and the end result is a region of the bond that is positive and part of the bond that is negative, thus making the bond polar in nature. Q: Why is sulfur dioxide polar and carbon dioxide non polar when both have polar covalent bond? Sulfur dioxide has a pungent smell, often likened to the smell of a match that has just been lit. Sulfur dioxide is naturally released by volcanic activity , and it is also present in the atmosphere due to the combustion of fossil fuels. This means that water is a polar molecule. It is a non-polar molecule. Much like the earth, molecules can have polar regions, but these polar regions are positive and negative in nature. Notify me of followup comments via e-mail. He aims to create content that educates, persuades, entertains and inspires. Is carbon dioxide polar or non- polar? Our panel of experts willanswer your queries. They are the ends of the molecules that have either a negative charge or positive charge, much like a battery has a negative end and a positive end. What about sulfur dioxide , is it polar or nonpolar?
To determine if SO 2 sulfur dioxide is polar or nonpolar, we need to first determine its geometry.
Photo: doctor-a via Pixabay, CC0. It is symmetric. The electron geometry, therefore, is trigonal planar , and the molecular geometry is bent. Sulfur dioxide is a polar molecules with polar covalent bonds. He aims to create content that educates, persuades, entertains and inspires. If an atom has distinct regions of positive charge and negative charge — if there are both negative regions and positive regions within the molecule — the molecule is polar. It is a non-polar molecule. When trying to determine the polarity of a molecule, you can use a three-step process to analyze it. What about sulfur dioxide , is it polar or nonpolar? This means that there is one side top or bottom of the molecule that has both oxygen atoms on it, which gives it a slightly negative charge while the portion of the molecule that has the sulfur atom has a slightly positive charge. So in essence, sulfur dioxide is polar while carbon dioxide is nonpolar because the individual movements of the bonds in carbon dioxide cancel one another out, yet in the case of sulfur dioxide, the angular nature of the molecule means that there is an imbalance between the poles — that it has both a negative and positive side — and therefore the molecule is polar. Much like H2O, sulfur is found in the middle of the molecule, with bent bonds connecting the sulfur to the oxygen. For instance, in carbon dioxide, the carbon-oxygen bonds are polarized toward the oxygen, which is more electronegative, and since both bonds have the same magnitude their sum is zero and the molecule is classified as nonpolar. Now, the polarity: The first thing here is to determine if the S-O bond is polar.

In it something is. Now all is clear, thanks for an explanation.
You commit an error. Write to me in PM, we will discuss.