Is pcl3 polar
Post a Comment.
Phosphorus compounds are very different and inflammable in nature. Phosphorus trichloride has the chemical formula PCl3. All atoms belong to the non-metal family group in the periodic table and possess high electronegativity values. In this blog post, we are going to discuss the polarity of PCl3 in a detailed manner. PCl3 is commonly appearing at ordinary temperatures and pressures, it exists as a liquid with a yellowish texture.
Is pcl3 polar
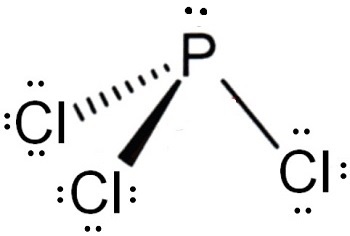
And how can you say that PCl3 is a polar molecule? Note: If you want to know the steps of drawing the PCl3 lewis dot structure, then visit this article: PCl3 lewis structure , Or you can also watch this short 2 minute video. And we also have to check the molecular geometry of PCl3. You can see the electronegativity values of Phosphorus P and Chlorine Cl atoms from the periodic table given below. Hence, the P-Cl bond is a polar covalent bond. But wait, we also have to look at the molecular geometry of PCl3 to know whether it has a symmetric shape or not. Have a look at this 3D structure of PCl3. The Phosphorus atom P is at the center and it is surrounded by 3 Chlorine atoms Cl. Because of this, there are positive and negative poles of charges on the overall molecule of PCl3. Is XeF4 Polar or Nonpolar?
The formal charge will be found on the central phosphorus atom of the PCl3 Lewis dot structure in the following calculation.
To determine if PCl 3 is polar or nonpolar, we need to first determine its geometry. This presumes knowing the rules for drawing a correct Lewis structure and you can find more details about Lewis structures here. Each chlorine taking three lone pairs leaves the nitrogen with one lone pair:. Therefore, the electron geometry is tetrahedral while the molecular geometry is trigonal pyramidal. Now, the polarity: The first thing here is to determine if the P-Cl bond is polar.
Phosphorus trichloride is a chemical substance known by its chemical formula PCl3. It exists as a colorless to yellow fuming liquid and considered to be toxic in nature. Many of you may have a question regarding whether PCl3 is a polar substance or not. In this article, I will answer this and will cover its properties and applications. PCl3 is a polar molecule because of its tetrahedral geometrical shape having a lone pair on Phosphorus atom and the difference between the electronegativity of Chlorine 3.
Is pcl3 polar
Phosphorus trichloride with a chemical formula PCl3 is a yellow fuming liquid. This liquid can be colorless as well. PCl3 is a toxic liquid with an unpleasant smell. The molar mass of this compound is The melting point and boiling point of this compound are Now there can be questions about the polarity of this compound.
Scalp scratcher
Is PCl3 polar or nonpolar, then? Is H2O Polar? Hyb can be calculated as follows. However since chlorine is not as electronegative as fluorine this effect is not as extreme. As a result of this, both two chlorine atoms form covalent bonds with the phosphorus atom, leaving the phosphorus atom with one lone pair. It can also be utilized in chemical reactions in order to add just chlorine to different structures such as alcohols. The lone pairs of phosphorus atom cause repulsion with P-Cl bond pairs, causing the P-Cl bonds to face downward force and the shape of the molecules to a tetrahedral pyramid like that of the NH3 ammonia molecule. Because the form of PCl3 is asymmetric. It is used in the production of plastics, pesticides, and other chemicals. Can hydroxypropyl methyl cellulose be used as an additive in animal feed?
Phosphorus trichloride is an inorganic compound with the chemical formula PCl 3. A colorless liquid when pure, it is an important industrial chemical , being used for the manufacture of phosphites and other organophosphorus compounds. It is toxic and reacts readily with water to release hydrogen chloride.
Second, the difference in electronegativity between phosphorus and chlorine atoms causes the P-Cl bonds to become polar, causing the entire molecule to become polar as well, resulting in a net dipole moment of the PCl3 molecule is 0. Furthermore, because chlorine is the most electronegative element in chemistry, it can never be the central atom in a PCl3 Lewis structure diagram. As a result, first, wrap around the leftover valence electrons on each chlorine atom. Phosphorus atom receives a partial positive charge on it, while three chlorine atoms receive a partial negative charge on it. It comes under the halogen family group in the periodic table. The formal charge will be found on the central phosphorus atom of the PCl3 Lewis dot structure in the following calculation. In closing, phosphorous trichloride is polar because it has a high electron density on the phosphorus atom and a low electron density on the chlorine atoms. You can also subscribe without commenting. The formation of a polar molecule is caused by the geometrical structure and the difference in electronegativity value of atoms in the PCl3 molecule. Is PCl3 Polar? Geometry PCl3 Polar or Nonpolar.

Quite right! It is good idea. I support you.
I apologise, I can help nothing. I think, you will find the correct decision.