Is h2o planar
And I acknowledge that English may not be your first language in which case you are doing well!
This theory states that as electrons are negatively charged, the valence electrons in different atoms in a molecule repel each other. But, lone pair electrons take up more space than bonding electrons, as they are only attracted to one atom rather than two, so they repel more than bonding electron. The carbon is in the centre because it has lower electronegativity. If we only form single bonds from C-O, carbon does not form a stable octet of electrons so we need to from double bonds. We cannot put hydrogen in the centre because it can only hold two electrons, due to its principle quantum number of 1. Therefore oxygen goes in the centre. Forming single bonds to each hydrogen leaves two more pairs of electrons which go around the oxygen atom, to complete the octet.
Is h2o planar
Is H 2 O 2 planar in structure? H 2 O 2 is stored in. In which of the following reactions H 2 O 2 acts as reducing agent? In which of the following reactions H 2 O 2 acts as a reducing agent? A : Dihedral angle of H 2 O 2 in gas phase is greater than in solid phase. R : H 2 O 2 has planar structure. Two structures of H 2 O 2 are drawn below. Identify the phases X and Y of H 2 O 2. For both cases its n-factor is 2. Statement H 2 O 2 is a polar covalent molcule. Strength of 10 volume hydrogen peroxide solution means. H 2 O 2 is always stored in black bottles because. Is H 2 O 2 planar in nature? The ionisation energy of hydrogen is high as compared to alkali metals
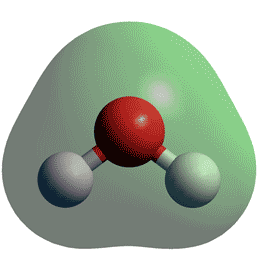
We did this by looking at a particular central atom. This means is h2o planar lone pairs are strong enough to repel the bonding pairs so that H2o results in the bent shape.
Molecular geometry, also known as the molecular structure, is the three-dimensional structure or arrangement of atoms in a molecule. Understanding the molecular structure of a compound can help determine the polarity, reactivity, phase of matter, color, magnetism, as well as the biological activity. To determine the shapes of molecules, we must become acquainted with the Lewis electron dot structure. Although the Lewis theory does not determine the shapes of molecules, it is the first step in predicting shapes of molecules. The Lewis structure helps us identify the bond pairs and the lone pairs.
Thus far, we have used two-dimensional Lewis structures to represent molecules. A bond angle is the angle between any two bonds that include a common atom, usually measured in degrees. A bond distance or bond length is the distance between the nuclei of two bonded atoms along the straight line joining the nuclei. Valence shell electron-pair repulsion theory VSEPR theory enables us to predict the molecular structure, including approximate bond angles around a central atom, of a molecule from an examination of the number of bonds and lone electron pairs in its Lewis structure. The VSEPR model assumes that electron pairs in the valence shell of a central atom will adopt an arrangement that minimizes repulsions between these electron pairs by maximizing the distance between them. The electrons in the valence shell of a central atom form either bonding pairs of electrons, located primarily between bonded atoms, or lone pairs. The electrostatic repulsion of these electrons is reduced when the various regions of high electron density assume positions as far from each other as possible.
Is h2o planar
H2O is the molecular formula of water, one of the major constituents of the Earth. A single molecule is made up of two hydrogen atoms and one oxygen atom, which are bonded through the covalent bond. Moreover, two or more H2O molecules connect with the help of hydrogen bonds to form a compound.
Elly clutch xxx
Ionisation energy of hydrogen is When drawing the Lewis Structure of H2O, it has 4 bonding regions tetrahedral geometry , 2 of which are lone pairs. These lone pairs produce a bent shape. See this older answer and links. The Lewis structure helps us identify the bond pairs and the lone pairs. Who is online Users browsing this forum: No registered users and 1 guest. For both cases its n-factor is 2. I think you are almost there The shape of CO 2 is linear because there are no lone pairs affecting the orientation of the molecule. Post by Chris Van 2J » Mon Nov 29, am H2O would be a bent shape as the lone pairs on the oxygen would push the hydrogens making it a bent shape with Post by Triston Dinh 1D » Tue Nov 09, pm The electron pair arrangement of H20 would be tetrahedral since it has a total of 4 regions of electron density 2 lone pairs and 2 bonding atoms. Post by michaelcrisera » Sun Nov 28, pm. Answered by Caroline W. Calculate oxidation number of Br in Br3O8.
Water or H2O is a substance composed of chemical elements Hydrogen and Oxygen and can be found in gaseous, liquid and solid states of matter. It is available in abundance as an essential element present in many compounds. One common question that arises among students is whether H2O water is polar or nonpolar.
The reaction between which of the following reactants produces hydroge Carbon dioxide is therefore linear in electron-group geometry and in molecular geometry. Post by oliviahelou » Fri Dec 03, am. The shape of CO 2 is linear because there are no lone pairs affecting the orientation of the molecule. By breaking the molecule into 4 parts each part looks at 1 of the 4 Carbons , we determine how many electron groups there are and find out the shapes. When the electron groups are all bond pairs, they are named exactly like the electron-group geometry. Summary of Dipole Moments To recap, when a molecule is polar it means that the electron is not distributed evenly and there is a difference in the electronegativity of the atoms. On the cross-base arrow, the cross represents the positive charge and the arrow represents the negative charge. Well, EN is how much an element really wants an electron. Now, let's refer back to tetrahedrals. Using the example above, we would add that H 2 O has a bond angle of High purity dihydrogen is obtained by electrolysing Question cf5ac. By checking the geometry of molecules chart above, we have a tetrahedral shape.

I consider, that you are not right. I am assured. Write to me in PM, we will communicate.
It is reserve
The matchless phrase, is pleasant to me :)