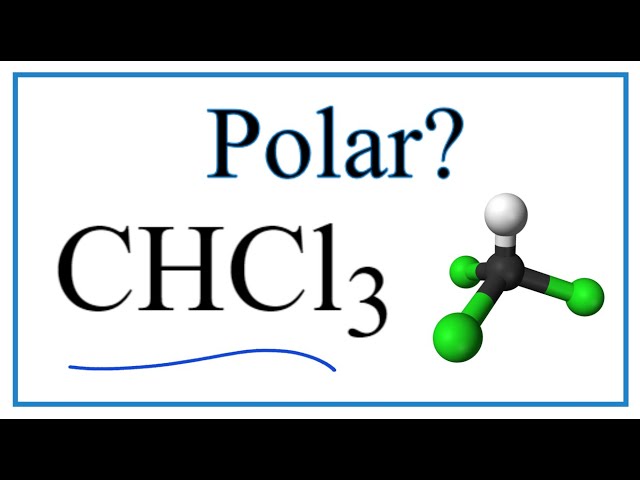
Is chcl3 polar or nonpolar
CCl4 is nonpolar because the bond polarity gets canceled with each other due to the symmetrical geometrical structure of its molecule. CHCl3 is polar due to its tetrahedral molecular structure and difference between the electronegativity of C, H and, CL.
Post a Comment. Answer: CHCl3 is a polar molecule due to the large electronegativity difference between hydrogen 2. This induces a permanent dipole across the molecule with a partial positive charge on the hydrogen atom and a partial negative charge on the chlorine atoms. Since the one hydrogen on the structure 2. This means that the compound is a liquid at standard temperature and pressure.
Is chcl3 polar or nonpolar
To determine if a molecule is polar or nonpolar, it is frequently useful to look at Lewis structures. Nonpolar compounds will be symmetric, meaning all of the sides around the central atom are identical - bonded to the same element with no unshared pairs of electrons. Another non polar molecule shown below is boron trifluoride, BF 3. BF 3 is a trigonal planar molecule and all three peripheral atoms are the same. Polar molecules are asymmetric, either containing lone pairs of electrons on a central atom or having atoms with different electronegativities bonded. This works pretty well - as long as you can visualize the molecular geometry. That's the hard part. Assuming you do, you can look at the structure of each one and decide if it is polar or not - whether or not you know the individual atom electronegativity. This is because you know that all bonds between dissimilar elements are polar, and in these particular examples, it doesn't matter which direction the dipole moment vectors are pointing out or in. A polar molecule is a molecule in which one end of the molecule is slightly positive, while the other end is slightly negative. As mentioned in section 4. The two electrically charged regions on either end of the molecule are called poles, similar to a magnet having a north and a south pole. A molecule with two poles is called a dipole see figure below. Hydrogen fluoride is a dipole.
Another non polar molecule shown below is boron trifluoride, BF 3. Water is a bent molecule because of the two lone pairs on the central oxygen atom. Oxygen is nonpolar.
.
Solvents used in organic chemistry are characterized by their physical characteristics. Among the most important are whether the solvents are polar or non-polar, and whether they are protic or aprotic. Because non-polar solvents tend to be aprotic,the focus is upon polar solvents and their structures. Solvents are generally classified by the polarity, and considered either polar or non-polar, as indicated by the dielectric constant. However, as with many properties, the polarity is a continuous scale, and the correct question is not "is it polar or non-polar" but "how polar is it. Generally, solvents with dielectric constants greater than about 5 are considered "polar" and those with dielectric constants less than 5 are considered "non-polar. The table above distinguishes between protic and aprotic solvents. For the solvents included in the table, the distinguishing feature is the presence of an -OH group, and that is the most common characteristic of a protic solvent.
Is chcl3 polar or nonpolar
If you're seeing this message, it means we're having trouble loading external resources on our website. To log in and use all the features of Khan Academy, please enable JavaScript in your browser. Donate Log in Sign up Search for courses, skills, and videos. About About this video Transcript.
Mlb app
Any molecule with lone pairs of electrons around the central atom is polar. This induces a permanent dipole across the molecule with a partial positive charge on the hydrogen atom and a partial negative charge on the chlorine atoms. Assuming you do, you can look at the structure of each one and decide if it is polar or not - whether or not you know the individual atom electronegativity. CHCl3 is polar due to its tetrahedral molecular structure and difference between the electronegativity of C, H and, CL. Comments No comments. Methanol is polar. No comments:. Give reasons. Explain with reason. How is CHCl3 utilized in the real world? Oxygen is nonpolar. Polar molecules are asymmetric, either containing lone pairs of electrons on a central atom or having atoms with different electronegativities bonded. A polar molecule is a molecule in which one end of the molecule is slightly positive, while the other end is slightly negative.
Trichloromethane, commonly known as chloroform, is a volatile organic compound in which one C-atom is covalently bonded to 3 Cl-atoms and 1 H-atom. At laboratory scale, it is prepared by chlorination of ethanol.
This means that the compound is a liquid at standard temperature and pressure. Contributors and Attributions StackExchange thomij. Methanol is polar. This induces a permanent dipole across the molecule with a partial positive charge on the hydrogen atom and a partial negative charge on the chlorine atoms. Created with MolView. Molecular Polarity To determine if a molecule is polar or nonpolar, it is frequently useful to look at Lewis structures. A polar molecule is a molecule in which one end of the molecule is slightly positive, while the other end is slightly negative. Your physics assignments can be a real challenge, and the due date can be really close — feel free to use our assistance and get the desired result. Sign in. Be the first! Nonpolar compounds will be symmetric, meaning all of the sides around the central atom are identical - bonded to the same element with no unshared pairs of electrons. Based on the periodic table only, which of the following options arranges the elements: Ar, Se, S,.

For a long time I here was not.