Is ch3oh hydrogen bonding
Serwis Infona wykorzystuje pliki cookies ciasteczka. Są to wartości tekstowe, zapamiętywane przez przeglądarkę na urządzeniu użytkownika.
Central European Journal of Chemistry. The molecules of both complexes in crystal structures are linked by O—H···O hydrogen bonds, which created a three-dimensional hydrogen-bonding networks. The π-π stacking interactions are also observed in crystal structures of complex 2. The spectral properties IR and electronic spectra of both complexes were also investigated. Copper complexes , carboxylates , supramolecular chemistry , hydrogen bonds , crystal structure.
Is ch3oh hydrogen bonding
PL EN. Skip to main menu Scroll to content. Polski English Language. Link to site. Open Chemistry. Article title. Self-assembled hydrogen-bonded coordination networks in two copper II carboxylates with 4-pyridylmethanol. Full texts:. Title variants. Languages of publication. The molecules of both complexes in crystal structures are linked by O-H…O hydrogen bonds, which created a three-dimensional hydrogen-bonding networks. The Π-Π stacking interactions are also observed in crystal structures of complex 2. The spectral properties IR and electronic spectra of both complexes were also investigated.
Chapter V. Książki na zamówienie. Wrocław, dnia 31 maja r.
This definitive reference consolidates current knowledge on dihydrogen bonding, emphasizing its role in organizing interactions in different chemical reactions and molecular aggregations. After an overview, it analyzes the differences between dihydrogen bonds, classical hydrogen bonds, and covalent bonds. It describes dihydrogen bonds as intermediates in intramolecular and intermolecular proton transfer reactions. It describes dihydrogen bonding in the solid-state, the gas phase, and in solution. This is the premier reference for physical chemists, biochemists, biophysicists, and chemical engineers.
Most people are comfortable with the idea of ionic and covalent bonds, yet unsure about what hydrogen bonds are, how they form, and why they are important. A hydrogen bond is a type of attractive dipole-dipole interaction between an electronegative atom and a hydrogen atom bonded to another electronegative atom. This bond always involves a hydrogen atom. Hydrogen bonds can occur between molecules or within parts of a single molecule. A hydrogen bond tends to be stronger than van der Waals forces , but weaker than covalent bonds or ionic bonds. However, even this weak bond is strong enough to withstand slight temperature fluctuation. How can hydrogen be attracted to another atom when it is already bonded? In a polar bond , one side of the bond still exerts a slight positive charge, while the other side has a slight negative electrical charge. Forming a bond doesn't neutralize the electrical nature of the participant atoms. Hydrogen bonds are found in nucleic acids between base pairs and between water molecules.
Is ch3oh hydrogen bonding
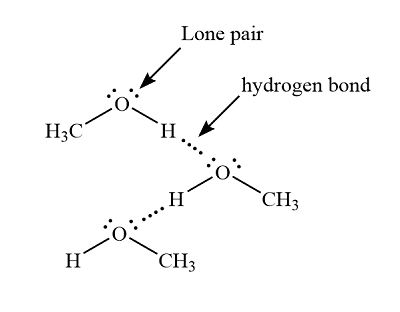
Like formaldehyde, methanol freezes somewhere around o C, but it does not become a gas until it is heated to 65 o C. Methanol is certainly similar to formaldehyde in some ways. It contains oxygen and is very polar. The huge difference in their boiling points is due to the very strong hydrogen bonds in methanol. Hydrogen bonding occurs when there is a significant amount of positive charge building up on a hydrogen atom. That happens because the hydrogen is attached to an atom that is much more electronegative than the hydrogen. As a result of that positive charge, a lone pair on another molecule strongly interacts with the hydrogen. Hydrogen bonds occur only when a hydrogen is attached to one of the few most electronegative elements in the periodic table: fluorine, oxygen or nitrogen.
Nikly onlyfan leak
Dni otwarte. Wysoki kontrast Włączony Wyłączony. Múdra, J. Zdeňka Padělková. Turn it on to take full advantage of this site, then refresh the page. Bernstein, R. Są to wartości tekstowe, zapamiętywane przez przeglądarkę na urządzeniu użytkownika. Camalli, G. Copper complexes Carboxylates Supramolecular chemistry Hydrogen bonds Crystal structure. Hudecová, M. Describes dihydrogen bonds as intermediates in intramolecular and intermolecular proton transfer reactions.
CH3OH is the molecular formula of methanol, also known as methyl alcohol, which is the simplest aliphatic alcohol.
Przypisz innemu użytkownikowi Wyszukaj użytkownika Zaproś. Polski English Zaloguj się lub załóż konto. Ucar, I. China, huainanweiyijun Główne kierunki badań. Languages of publication. Soc, , Musisz się zalogować, aby powiadomić znajomego e-mailem Zaloguj się lub załóż konto. Intramolecular dihydrogen bonds C—H? Segľa, D. Beatty, Coord. Konferencje dla szkół.

I am assured, what is it already was discussed, use search in a forum.
I think, that you commit an error. I can prove it. Write to me in PM, we will communicate.
It is removed