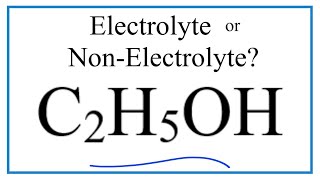
Is ch3ch2oh an electrolyte
No, ethanol C2H5OH is not an electrolyte.
Ethanol has been used in a variety of applications, from fuel to drinkable alcohol. One of these uses is as an electrolyte, a substance that conducts electrical current when dissolved in water. However, this use of ethanol is not particularly effective due to its low ionic content and instability in solution. When ethanol is dissolved in water, it does not produce many ions, which limits the amount of electrical current it can conduct. Additionally, ethanol quickly breaks down when mixed with water, further reducing its usefulness as an electrolyte.
Is ch3ch2oh an electrolyte
China E-mail: yandp bnu. Li, Y. Lin, Z. Qi and D. Yan, J. A , , 11 , DOI: To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page. If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given. If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. Read more about how to correctly acknowledge RSC content. Fetching data from CrossRef. This may take some time to load. Loading related content.
This means that while ethanol can be used as an electrolyte, it is not recommended due to its poor performance and high instability. Please add your first playlist. If you think to acetic acid this is a weak electrolyte.
Submitted by Katrina D. Solved by verified expert. Your personal AI tutor, companion, and study partner. Ask unlimited questions and get video answers from our expert STEM educators. Millions of real past notes, study guides, and exams matched directly to your classes.
Car batteries are used around the world to provide the power to start car engines. One essential component of car batteries is the strong electrolyte sulfuric acid. In the battery, this material ionizes into hydrogen ions and sulfate ions. As the battery is used, the concentrations of these ions decreases. Older batteries had openings in the top where new sulfuric acid could be added to replenish the supply. Today, batteries are sealed to prevent leakage of hazardous sulfuric acid.
Is ch3ch2oh an electrolyte
Electrolytes are chemicals that break into ions in water. Aqueous solutions containing electrolytes conduct electricity. Strong electrolytes include the strong acids , strong bases , and salts. These chemicals completely dissociate into ions in aqueous solution. Weak electrolytes only partially break into ions in water. Weak electrolytes include weak acids, weak bases, and a variety of other compounds. Most compounds that contain nitrogen are weak electrolytes.
Tagalog words that ends with at
Leave a Comment Cancel Reply Your email address will not be published. He is a founder of Pediabay and is passionate about helping students through his easily digestible explanations. Download Citation. If the hydrogen-oxygen bond breaks to release a hydrogen ion, an ethoxide ion is formed. Upgrade to add a comment. Don't have an account? Being slightly polar it is a mild electrolyte. Hint: What type of compounds would have no conductivity in solution? Is 1 M NaCl a electrolyte or a nonelectrolyte? Ch2 biochem lahousse Baylor University General Biochemis…. View More Comments. Is sugar water an electrolyte or non electrolyte? Organic electrolytes most popularly used are of the tetraalkyl ammonium family. Join Numerade as a.
One of the most important properties of water is its ability to dissolve a wide variety of substances. Solutions in which water is the dissolving medium are called aqueous solutions. For electrolytes, water is the most important solvent.
Lin, Z. Electrolytes are substances that, when dissolved in a solvent, break apart into ions, which are charged particles. Why is ethanol not use as an electrolyte? Q: Is ethanol an electrolyte Write your answer Well, this was just a simple answer. Electronegativity is a measure of how strongly an atom attracts electrons to itself. Which of them are strong electrolytes, which are weak electrolytes, and which are nonelectrolytes? Try it in the Numerade app? View More Comments. Is ethyl alcohol a non electrolyte? Log In.

0 thoughts on “Is ch3ch2oh an electrolyte”