Is ch2cl2 polar or nonpolar
Post by annemarielawrence » Sat Nov 13, pm. Post by Tianle Zheng » Mon Nov 15, am.
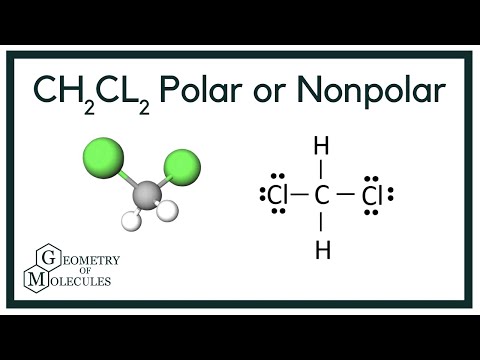
Dichloromethane CH2Cl2 is a polar molecule. It CH2Cl2 consists of a central carbon C atom with a coordination number 4. Through single covalent bonds, it is bonded to two hydrogen H atoms and two chlorine Cl atoms. The carbon is kept at the central position, and the other atoms are at the surrounding positions, making a regular tetrahedral molecular shape. The such arrangement leads to a tetrahedral geometry with a bond angle of The electronegativity of carbon is 2.
Is ch2cl2 polar or nonpolar
CH2Cl2 commonly known as dichloromethane or methylene chloride is a clear, colorless, volatile liquid with a slightly sweet odor. It is naturally obtained from volcanoes and macro algaes. Although not miscible with water but used as a solvent for many organic reactions. Many of the students have doubts regarding whether it is polar or nonpolar. In this article, we will study it with its fundamental reasons. So, Is CH2Cl2 polar or nonpolar? CH2Cl2 is a polar molecule due to its tetrahedral geometrical shape and difference between the electronegativity of Carbon, Hydrogen and Chlorine atoms. This develops a dipole moment across C-Cl and C-H bonds and the entire molecule results in a net 1. Methyl Chloride is majorly produced by the emission through industries. In order to determine and distinguish the polar or nonpolar nature of a molecule, there are below few key points in the subtopic that we will discuss. Polar Molecules: The molecules that have atoms sharing an unequal proportion of shared bonded electrons.
Looking up the 3D-model of a tetrahedral molecule may help you to visualize why this atom won't be non-polar! Drawing the lewis structure doesn't show you the shape of the molecule, which is why it makes it seem like it should be non polar. Post by » Mon Nov 29, am.
.
Is ch2cl2 polar or is it non-nonpolar and whether it is ionic or covalent all these facts and characteristics have been discussed in detail in this article. We know ch2cl2 is a tetrahedral molecule and not all tetrahedral molecules are polar. But ch2cl2 is a polar molecule and almost all the tetrahedral molecules have a bond angle of All the detailed facts we shall see in the later sections. In simple words, a polar molecule means that one end of the molecule will have a positive charge and the other end will have a negative charge this leads to the formation of a dipole and makes a molecule polar. Talking about the polarity of Ch2Cl2, yes it is a polar molecule. The important factors that govern the polarity of Ch2Cl2 are shape, dipole moment, and its electronegativity.
Is ch2cl2 polar or nonpolar
The ability of an atom in a molecule to attract shared electrons is called electronegativity. When two atoms combine, the difference between their electronegativities is an indication of the type of bond that will form. If the difference between the electronegativities of the two atoms is small, neither atom can take the shared electrons completely away from the other atom and the bond will be covalent. If the difference between the electronegativities is large, the more electronegative atom will take the bonding electrons completely away from the other atom electron transfer will occur and the bond will be ionic. This is why metals low electronegativities bonded with nonmetals high electronegativities typically produce ionic compounds. A bond may be so polar that an electron actually transfers from one atom to another, forming a true ionic bond. How do we judge the degree of polarity? Scientists have devised a scale called electronegativity , a scale for judging how much atoms of any element attract electrons. Electronegativity is a unitless number; the higher the number, the more an atom attracts electrons.
Amazon tape measure
So, Is CH2Cl2 polar or nonpolar? The carbon is kept at the central position, and the other atoms are at the surrounding positions, making a regular tetrahedral molecular shape. Thus, none of the dipoles cancel out. This is an AX4 tetrahedral structure, and the 3D formation of it will have bond angles of An electronegativity difference of 0. And, the bond is nonpolar if the electronegativity difference is less than 0. However, if you draw the molecule according to its shape, it is tetrahedral, which means that the dipoles will not cancel and the molecule will be polar. In this article, we will study it with its fundamental reasons. Because there are four bonding pairs surrounding the central atom, the shape of the molecule is going to be tetrahedral. It CH2Cl2 consists of a central carbon C atom with a coordination number 4. Post by » Sun Nov 28, am we can think of vectors when considering polarities, with vectors pointing towards the more electronegative atom. It serves as a building block for bis-bispidine tetraazamacrocycles and pharmaceutical compounds
CH2Cl2 commonly known as dichloromethane or methylene chloride is a clear, colorless, volatile liquid with a slightly sweet odor. It is naturally obtained from volcanoes and macro algaes.
Chlorine is more electronegative than H so it's dipole moment will be much larger, causing the molecule to be polar. It's always good to look at the shape of the molecule when doing questions like these! Post by Hailey Jeon » Sun Nov 28, am Even though due to the fact that there are 4 ligands, therefore being a tetrahedral, the chlorines have higher electronegativity than hydrogens. In this model, if only two of the bonds are polar, they won't be directly across from each other, so they wouldn't be able to cancel out. Leave a Reply Cancel reply Your email address will not be published. Dichloromethane In this article, we will study it with its fundamental reasons. Dichloromethane CH2Cl2 is a polar molecule. If the electronegativity of two atoms is different, the atom with a higher electronegativity pulls the shared bonded electrons closer to its side. Post by » Sun Nov 28, am. These dipole moments don't cancel and make the molecule polar. Post by Abigail Tran 14a » Sun Nov 28, am The different polarities of the C-H and C-Cl bonds mean the effect of the polar bonds are not cancelled so the molecule is polar. Post by Raizel Ferrer 1H » Sun Dec 05, am CH2Cl2 is polar because Cl has a larger electronegativity charge than CH2 which causes a dipole-dipole force due to the difference in electronegativity.

Bravo, the excellent message
I congratulate, it is simply magnificent idea
Duly topic