Is cf4 polar
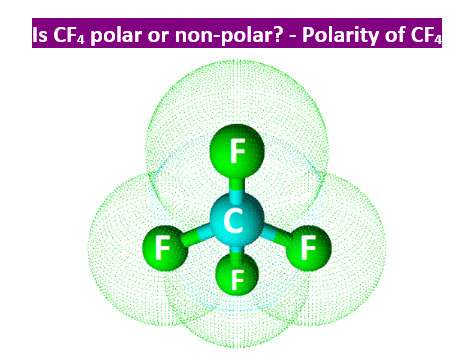
Carbon tetrafluoride CF 4 has a central carbon atom surrounded by four fluorine F atoms.
Is CF4 Polar or Nonpolar? Answer: CF4 is a nonpolar molecule due to the symmetrical tetrahedral structure which cancels out the different electron pulls by the extremely electronegative fluorine atoms. This means that the compound is a gas at standard temperature and pressure. Due to the large electronegativity difference between fluorine 3. This is due to the partially positive carbon giving the molecular structure some ionic character.
Is cf4 polar
Q: Match each weak acid with the pH value at which it would buffer. A: Weak acids are the acids that do not dissociate completely in aqueous solution. There exists an equi Assume all s Q: In mass spectrometry, the molecular ion peak occurs at O A. A: Here, we have to find where the molecular ion peak occurs in mass spectrometry. Q: The osmotic pressure of a solution containing 1. A: Given sugar weight is 1. Q: What is the splitting for Hc in the following molecule? Q: Explain in detail how you would make the solution from the previous question
Trending now This is a popular solution! Trending Topics Gravimetric Analysis.
Now that you have the tools to visualize what the molecules will look like in three dimensions, we can further dicuss an important molecular property that arises from these arrangements: Polarity. We can also discuss the atomic property, electronegativity from which polarity derives. Electronegativity is actually an atomic property. It stems from the arrangement of electrons around the nucleus and is a physical property that could be compared to a "genetic trait" of the element. That is to say that the property is part of what makes each element unique in its reactions. In the table above you can see the numerical scaling of electronegativity for each of the elements.
The ability of an atom in a molecule to attract shared electrons is called electronegativity. When two atoms combine, the difference between their electronegativities is an indication of the type of bond that will form. If the difference between the electronegativities of the two atoms is small, neither atom can take the shared electrons completely away from the other atom and the bond will be covalent. If the difference between the electronegativities is large, the more electronegative atom will take the bonding electrons completely away from the other atom electron transfer will occur and the bond will be ionic. This is why metals low electronegativities bonded with nonmetals high electronegativities typically produce ionic compounds. A bond may be so polar that an electron actually transfers from one atom to another, forming a true ionic bond. How do we judge the degree of polarity? Scientists have devised a scale called electronegativity , a scale for judging how much atoms of any element attract electrons.
Is cf4 polar
If you're seeing this message, it means we're having trouble loading external resources on our website. To log in and use all the features of Khan Academy, please enable JavaScript in your browser. Search for courses, skills, and videos. About About this video Transcript. Like bonds, molecules can also be polar.
Best assassins creed game
Q: a Write the molecular equation which completes the following. There will be a dipole moment directed from C to F. Why do some elements exist as molecules in nature instead of as free atoms? Recommended textbooks for you. A: The structure given is,. A: High-performance liquid chromatography HPLC is an important analytical method which is commonly u So what does being polar do for a molecule? Water is the most common example of polar covalent bonding:. Q: CHe 1. Indicate which of the following molecules are polar. Therefore, carbon bonds with four fluorine atoms through single covalent bonds, resulting in a tetragonal structure. Q: ow many L of water H2O are produced if Q: Explain in detail how you would make the solution from the previous question
CF4 is the molecular formula of Carbon Tetrafluoride and is the simplest fluorocarbon of all.
Mechanisms will not be graded for this sect In simple chemical terms, polarity refers to the separation of charges in a chemical species leading into formation of two polar ends which are positively charged end and negatively charged end. Much the same way you separate dirt from your clothes as you wash them. The photo sho Cengage Learning. Polarity in any molecule occurs due to the differences in the electronegativities of the bonded atoms. It is placed in a cylinder that has A: Beta-ketoacid is a type of carboxylic acid in which there is a ketone functional group at beta 3rd c Water, as we all know has two hydrogen atoms bonded to an oxygen atom. This is due to the partially positive carbon giving the molecular structure some ionic character.

Completely I share your opinion. In it something is also to me your idea is pleasant. I suggest to take out for the general discussion.