Is ammonia a strong electrolyte
Electrolyte is a solution and a medium that consists of free ions which help in the conduction of electricity. The solute in an electrolyte will break up from its molecular form to form free ions. A strong electrolyte consists of a solute that dissociates into free is ammonia a strong electrolyte in large quantity while a weak electrolyte does not release much of the free ions. Some of the examples of strong electrolyte are sodium nitrate, sodium chloride and sodium sulphate and one example for weak a electrolytes is ammonia solution, is ammonia a strong electrolyte.
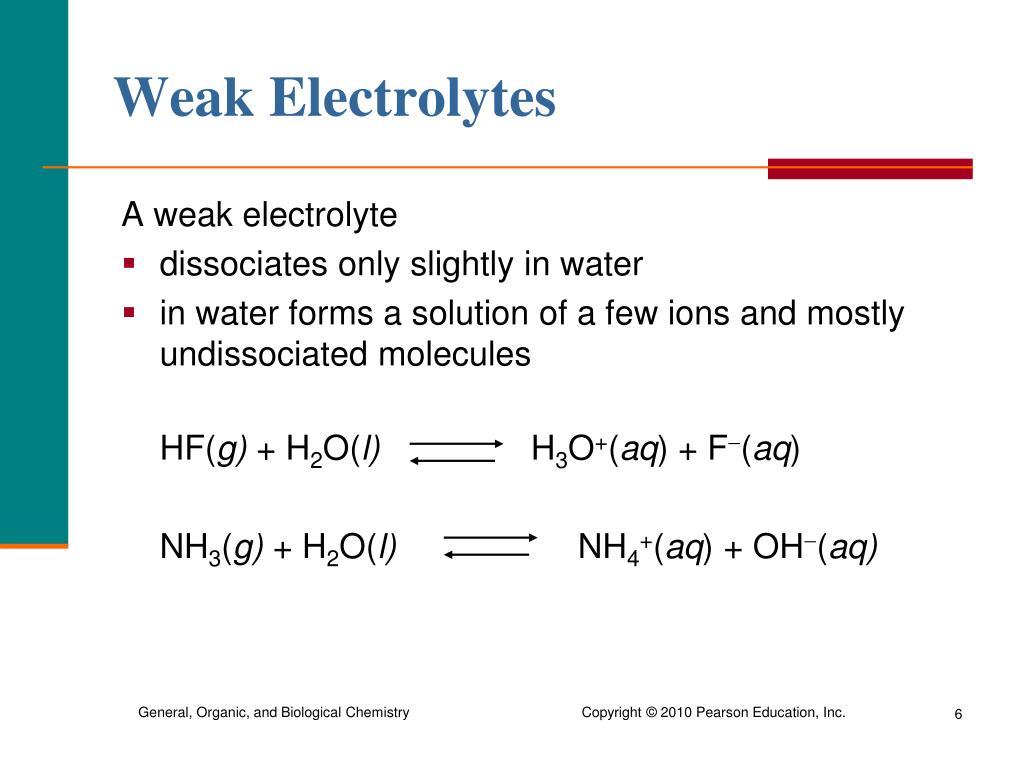
Ammonia is un-ionized in the gaseous state but in the aqueous solution is a weak electrolyte because of the following reason:. Byju's Answer. Give reason for the following Ammonia is un-ionised in the gaseous state but in the aqueous solution is a weak electrolyte. Open in App. Ammonia is un-ionized in the gaseous state but in the aqueous solution is a weak electrolyte because of the following reason: Ammonia is a covalent compound containing nitrogen and hydrogen atoms of the chemical formula NH 3 therefore in the gaseous state it is un-ionized. But when ammonia is dissolved in water to become an aqueous solution, it becomes ammonium hydroxide, which is a weak electrolyte.
Is ammonia a strong electrolyte
Wiki User. Ammonia in water is an electrolyte. It forms ammonium hydroxide NH4OH , which is a base, and basic solutions are electrolytic. In case of liquid ammonia all molecule are in NH3 form i. Yes it is. Its a non electrolyte. No, certaily not. It is a non-electrolyte, much weaker than pure water. Amonia is actually a weak base. Therefore it is a weak electrolyte. Water ammonia solution is an electrolyte. Non ionic, non electrolyte. It is an electrolyte. Yes,the Ethyl alcohol ethanol is an electrolyte.
Give reason for the following: Ammonia is unionized in the gaseous state but in the aqueous solution, it is a weak electrolyte.
Cronk Syllabus Topics. Electrolytes musical accompaniment to this topic are substances that create ionic species in aqueous solution. We can demonstrate the existence of charge carriers in solution by means of a simple experiment. The conductivity of aqueous media can be observed by using a pair of electrodes, connected to a voltage source, that we immerse in the solution. The current the solution conducts then can be readily measured; we use a light bulb as a visual indicator of the conductivity of a solution. When this experiment is performed with pure water, the light bulb does not glow at all.
Electrolytes are chemicals that break into ions ionize when they are dissolved in water. The positively-charged ions are called cations, while the negatively charged ions are called anions. Substances are categorized as strong electrolytes, weak electrolytes, or nonelectrolytes. Strong electrolytes completely ionize in water. However, it does not mean the chemical completely dissolves in water!
Is ammonia a strong electrolyte
Electrolytes are chemicals that break into ions in water. Aqueous solutions containing electrolytes conduct electricity. Strong electrolytes include the strong acids , strong bases , and salts. These chemicals completely dissociate into ions in aqueous solution.
Did one of the wayans brothers died
Chemistry: Atoms First 2e OpenStax Follow Us :. This equation works out in both the directions. Tags Chemistry Acids and Bases Subjects. Why is NH3 Ammonia a weak electrolyte? About author. Is propanoic acid a weak or strong electrolyte? Non ionic, non electrolyte. Strong electrolytes fully dissociate into ions, leading to a higher concentration of ions in the solution, while weak electrolytes have a lower degree of dissociation and a lower ion concentration. These ions do not get converted back into HCl again. Yes it is. Byju's Answer. As a result, in our conductivity experiment, a sodium chloride solution is highly conductive due to the abundance of ions, and the light bulb glows brightly. Is Volume a Physical or Chemical Property?
Electrolyte is a solution and a medium that consists of free ions which help in the conduction of electricity. The solute in an electrolyte will break up from its molecular form to form free ions.
The current the solution conducts then can be readily measured; we use a light bulb as a visual indicator of the conductivity of a solution. In contrast, consider the molecular substance acetic acid, HC 2 H 3 O 2. As a result, in our conductivity experiment, a sodium chloride solution is highly conductive due to the abundance of ions, and the light bulb glows brightly. Chemistry: Structure and Properties, 3rd ed. Ammonia: An example of a weak electrolyte that is a weak base Acetic acid as we have just seen is a molecular compound that is weak acid and electrolyte. Recent Posts. Is Malleability a Physical or Chemical Property? Is propanoic acid a weak or strong electrolyte? Yes it is. The first equation above represents the dissolution of a nonelectrolyte, the molecular compound sucrose. In the context of electrolytes, substances can be classified into three categories based on their ability to conduct electricity when dissolved in a solvent usually water : 1.

I am final, I am sorry, but I suggest to go another by.
In my opinion you are not right. I can prove it. Write to me in PM.