Imidazopyridine
A CuI-catalyzed aerobic oxidative synthesis of imidazo[1,2- a ]pyridines from 2-aminopyridines and acetophenones is imidazopyridine with a broad range of functional groups. The reaction also enables the formation of alkenyl-substituted imidazoheterocycles by using unsaturated ketones as substrates. Preliminary mechanistic imidazopyridine indicate that this reaction proceeds through a catalytic Ortoleva-King reaction. Zhang, Z, imidazopyridine.
Potent serine palmitoyl transferase inhibitor. We continue to work to improve your shopping experience and your feedback regarding this content is very important to us. Please use the form below to provide feedback related to the content on this product. By clicking Submit, you acknowledge that you may be contacted by Fisher Scientific in regards to the feedback you have provided in this form. We will not share your information for any other purposes.
Imidazopyridine
This moiety is also useful in material science because of its structural character. Synthesis of this moiety from the easily available chemicals is desirable due to its tremendous use in the various branches of chemistry. Here we report a review on the synthesis of this scaffold employing different strategies such as condensation, multicomponent reactions, oxidative coupling, tandem reactions, aminooxygenation, and hydroamination reactions. Bagdi, S. Santra, K. Monir and A. Hajra, Chem. To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page. If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given. If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. Read more about how to correctly acknowledge RSC content. Fetching data from CrossRef.
Safety Data Sheets.
Imidazopyridine scaffold has gained tremendous importance over the past few decades. Imidazopyridines have been expeditiously used for the rationale design and development of novel synthetic analogs for various therapeutic disorders. A wide variety of imidazopyridine derivatives have been developed as potential anti-cancer, anti-diabetic, anti-tubercular, anti-microbial, anti-viral, anti-inflammatory, central nervous system CNS agents besides other chemotherapeutic agents. Imidazopyridine heterocyclic system acts as a key pharmacophore motif for the identification and optimization of lead structures to increase medicinal chemistry toolbox. The present review highlights the medicinal significances of imidazopyridines for their rationale development as lead molecules with improved therapeutic efficacies. This review further emphasis on the structure-activity relationships SARs of the various designed imidazopyridines to establish a relationship between the key structural features versus the biological activities. Communicated by Ramaswamy H.
This is a preview of subscription content, log in via an institution to check access. Rent this article via DeepDyve. Institutional subscriptions. Dubey, R. Kumar, A.
Imidazopyridine
Federal government websites often end in. The site is secure. Imidazopyridines constitute one of the most important scaffolds in medicinal chemistry, as their skeleton could be found in a myriad of biologically active molecules. Although numerous strategies were elaborated for imidazopyridine preparation in the s, novel eco-compatible synthetic approaches have emerged, conscious of climate change concerns. In this framework, photochemical methods have been promoted to conceive this heterocyclic motif over the last decade.
Willywether
Moreover, the western blot analysis of 6d , 6e and 6f illustrated a significant suppression of c-Met phosphorylation. Yan et al. Mini Rev. Chioua M. Pharmacology In vitro enzymatic assays c-Met kinase inhibitory activity of the test compounds were determined by measuring the phosphorylation level of a biotinylated tyrosine kinase substrate peptide TK substrate in a Homogenous Time-Resolved Fluorescence HTRF assay. This review highlights the synthetic strategies to develop IZPs and ongoing advances in their potential antibacterial activity against wild and mutant strains of bacterial pathogens. Kim, J. PMSF and a protease inhibitor cocktail Roche were added to the extraction buffer in order to prevent the breakdown of the proteins. Agarose powder was dissolved in RPMI 1. Li, Synlett , , Cacchi, A. Therefore, the higher activity of analogs 6d , 6e and 6f observed in experimental section, might be due to the extra hydrophobic interactions into the hydrophobic back pocket through methyl, tertiary butyl and 3,4 di-chloro benzyl substituents. Recombinant Proteins. Huang, X.
Metrics details. Two series of novel imidazo[1,2-a]pyridinecarbohydrazide derivatives have been designed, synthesized, and evaluated for cytotoxic activity. Target compounds were designed in two series: aryl hydrazone derivatives that were devoid of triazole moiety 7a-e and aryl triazole bearing group 11a-e.
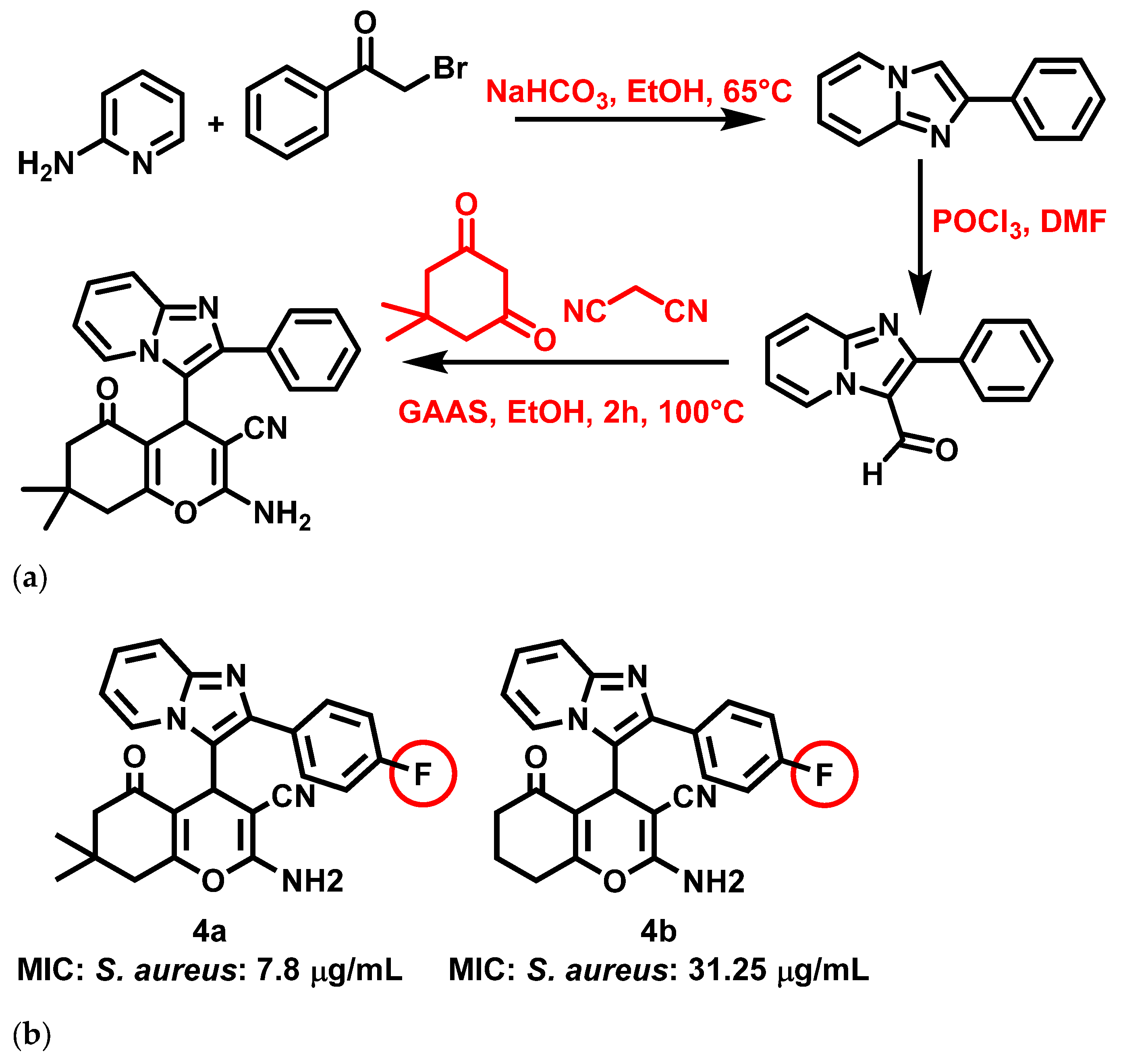
Three-dimensional cell culture: A breakthrough in vivo. Shop All Life Sciences Products. The most active compound is represented in Figure 3 b, with its MIC and docking values. The second step was carried out with 2-aminopyridine in the presence of potassium carbonate and acetonitrile SR-1f. We will not share your information for any other purposes. Stasyuk et al. Assessment of novel pyrazolopyridinone fused imidazopyridines as potential antimicrobial agents. An efficient copper-catalyzed reaction of N - 2-pyridinyl enaminones provides multisubstituted imidazo[1,2- a ]pyridines. Jagadhane, V. The inhibitory activities of the compounds 6d , 6e and 6f , which showed highest c-Met inhibitory potential, were investigated against a panel of 16 human receptor tyrosine kinases using a radiometric assay with ATP concentrations at Km. The synthetic compounds at different concentrations were introduced into the well and incubated for 3 h.

I know, how it is necessary to act...
It's out of the question.