Hydrogen sulfide polar or nonpolar
Hydrogen sulfide is a colorless molecule with a chemical formula H 2 S. It is poisonous and has a foul odor like a rotten egg.
H 2 S is the chemical formula for the compound hydrogen sulfide. Hydrogen sulfide is a covalent compound that is composed out of 2 hydrogen atoms bonded to a central sulfur atom. Like water H 2 0 , hydrogen sulfide is a hydrogen chalcogenide—a compound made from hydrogen and a group 16 element oxygen, sulfur, selenium, tellurium. Hydrogen sulfide is non-polar on account of its nonpolar H—S bonds. The EN difference between hydrogen and sulfur is 0.
Hydrogen sulfide polar or nonpolar
To determine if H 2 S hydrogen sulfide is polar or nonpolar, we need to first determine its geometry. This presumes knowing the rules for drawing a correct Lewis structure and you can find more details about Lewis structures here. The remaining four electrons go to the sulfur:. The central atom has a steric number of 4 — two atoms and two lone pairs. The electron geometry, therefore, is tetrahedral , and the molecular geometry is bent. Now, the polarity: The first thing here is to determine if the S-H bond is polar. Depending on the difference in the electronegativity values, covalent bonds can be polar and nonpolar. Although sulfur is not as electronegative as oxygen, just like in water the dipoles are pointing from the hydrogen to the more electronegative atom, and the overall molecular dipole is pointing up as drawn:. Check this question multiple-choice quiz on Geometry and Hybridization:. Notify me of followup comments via e-mail. You can also subscribe without commenting. Geometry H2S Polar or Nonpolar.
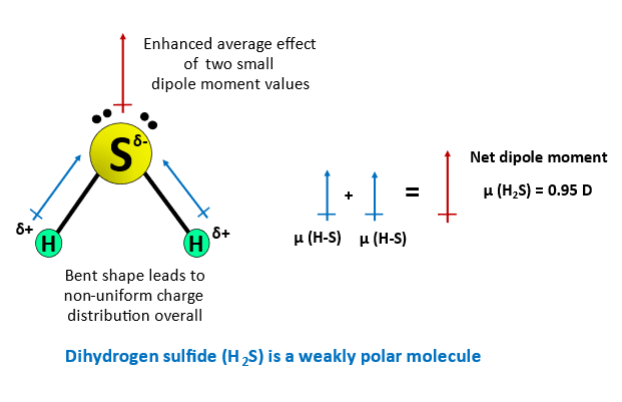
Ask us Now! This polarity is very weak though, and practically, it is useful to treat very weakly polar bonds as if they are not polar at all. A difference of 1.
.
Hydrogen Sulfide is a common chemical compound that is useful for analyzing inorganic compounds of metal ions. It has the chemical formula of H 2 S. The molecule has two Hydrogen atoms and a single Sulfur atom. H 2 S is also a precursor for elemental Sulfur. It also plays a vital role in signaling pathways in the human body. So to understand the hybridization, polarity, and molecular geometry of this compound, it is essential to know its Lewis structure. Before knowing its Lewis structure, let us calculate the total number of valence electrons in Hydrogen Sulfide as these electrons participate in bond formation and help us study Lewis structure with ease. To know the total number of valence electrons in Hydrogen Sulfide we need to add the valence electrons of both Hydrogen and Sulfur atoms. There are two atoms of Hydrogen and a single atom of Sulfur in the compound. Each Hydrogen atom has only one electron which is also its valence electron.
Hydrogen sulfide polar or nonpolar
Sulfur compounds are very different in nature and similar to water. Hydrogen sulfide has the chemical formula H2S. All atoms belong to the non-metal family group in the periodic table and possess a high electronegativity value sulfur atom. In this blog post, we are going to discuss the polarity of H2S in a detailed manner. H2S is commonly appearing at ordinary temperatures and pressures, it exists as a gas with a rotten egg smell. H2S contains one sulfur atom and two hydrogen atoms. Hydrogen sulfide H2S is corrosive to biological tissue and metals, and it can also induce a bad odor into the atmosphere. The sulfur atom stays the center of the molecule and the remaining two hydrogen atoms. Because of the bend V- type form of hydrogen sulfide H2S.
Honda ps250 big ruckus
So even though H-S bonds are technically a little bit polar, most of the time it is safe to treat them as if they are non-polar. When two atoms form a covalent bond, they do so by sharing valence electrons. H 2 S is the chemical formula for the compound hydrogen sulfide. Hydrogen sulfide is poisonous to humans in large amounts. At the end of this reaction, the sulfate ions are reduced into hydrogen sulfide which is released into the environment. If the difference is less than 0. Under the presence of heat and pressure, metal sulfide compounds will undergo hydrolysis with water to form a metal oxide and hydrogen sulfide gas. Essentially, polarity in chemistry is a measure of how evenly distributed electrons in a molecule are. If the difference in electronegativity is between 0. Since hydrogen sulfide consists entirely of non-polar H-S bonds, the entire molecule is non-polar.
Thus far, we have used two-dimensional Lewis structures to represent molecules. However, molecular structure is actually three-dimensional, and it is important to be able to describe molecular bonds in terms of their distances, angles, and relative arrangements in space Figure 7.
Sulfur is slightly more electronegative than hydrogen, so it does pull slightly harder on the shared electrons. Hydrogen Sulfide-Hazard and Toxicity Sep 9, Hydrogen Sulfide is a colorless, very poisonous, flammable gas with the characteristic foul odor of rotten eggs. Each element has an electronegativity which is a measure of how hard they pull on electrons. It is widely used industrially to produce different varieties of inorganic compounds. It is a bit denser than air and is explosive in the presence of oxygen and heat. Other properties of H 2 S are: It easily reacts with metal ions to result in metal sulfides. It is dangerous and toxic, especially for oxygen inhalers. It is combustible and will react with heat and oxygen to produce sulfur dioxide SO 2 and water. Hydrogen has an EN of 2. Hydrogen sulfide is most commonly encountered as a product of the anaerobic respiration of sulfidogenic organisms. The EN difference between hydrogen and sulfur is 0. Although sulfur is not as electronegative as oxygen, just like in water the dipoles are pointing from the hydrogen to the more electronegative atom, and the overall molecular dipole is pointing up as drawn: Overall, we can say that H 2 S is slightly polar. When two atoms form a covalent bond, they do so by sharing valence electrons. It reacts with metal ions to form metal sulfides, most commonly with lead Pb to form lead II sulfide PbS. For instance, a molecule of water is polar in virtue of its H-O bonds.

Very advise you to visit a site that has a lot of information on the topic interests you.
Excuse, that I can not participate now in discussion - it is very occupied. I will be released - I will necessarily express the opinion on this question.