Hybridization of carbon in co2
In this article, we will delve into the intriguing world of chemistry to explore the hybridization of CO 2. Carbon dioxide is an interesting molecule, with carbon hybridization of carbon in co2 its core exhibiting sp hybridization. This hybridization arises due to the carbon atom being bonded to two other atoms, which can be either two double bonds or a combination of one single and one triple bond. We can understand this better by scrutinizing each atom of CO 2 more closely.
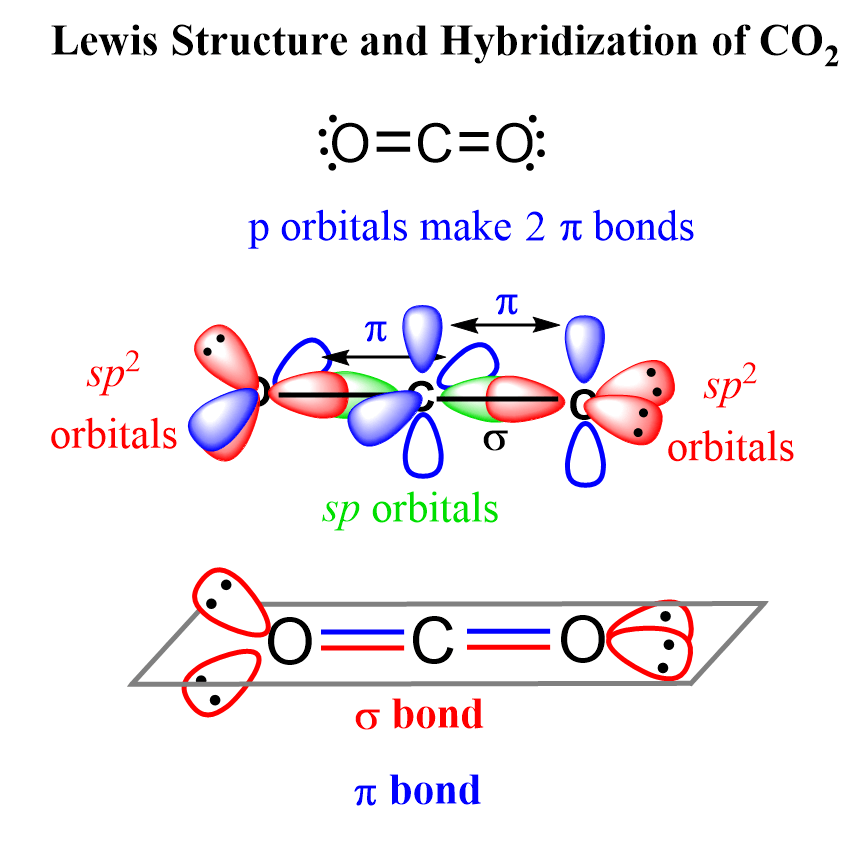
The carbon dioxide or CO2 has sp type hybridisation. This type of hybridisation occurs as an outcome of the carbon being bound to two different atoms. The atom of the carbon comprises 2 double bonds, i. However, this is not sufficient for creating bonds involving the oxygen. Therefore, one electron from the 2s orbital shifts from the 2s level to 2p level, which leads to the creation of 2 hybrid orbitals. These hybridised sp orbitals belonging to the carbon atoms extend beyond 2p orbitals that belong to the atoms of oxygen for creating two sigma bonds. A pi-bond is formed between the 2 leftover p electrons.
Hybridization of carbon in co2
We will learn about the hybridization of CO 2 on this page. Carbon dioxide basically has a sp hybridization type. This type of hybridization occurs as a result of carbon being bound to two other atoms. We can determine this by closely observing each atom of CO 2. In determining the hybridization of carbon dioxide, we will take the carbon atom first. The carbon atom has two effective pairs or two double bonds exist in it. However, this is not enough to form bonds with oxygen. What happens next is that one electron from 2s orbital moves from the 2s level to 2p level which results in the formation of two hybrid orbitals. Now, these sp hybridized orbitals of the carbon atom overlap with two p orbitals of the oxygen atoms to form 2 sigma bonds. As for the two remaining p electrons they will be used to form a pi bond. In carbon dioxide molecule, oxygen also hybridizes its orbitals to form three sp 2 hybrid orbitals. The p orbital in oxygen remains unchanged and is mainly used to form a pi bond.
How to calculate the hybridisation?
To determine the hybridization of carbon dioxide, let us take the carbon atom first. The carbon atom has two double bonds, or two effective pairs exist in it. However, this is not enough to produce bonds with oxygen. So, then, one electron from 2s orbital moves from the 2s level to the 2p level that results in the formation of two hybrid orbitals. Now, these hybridized sp orbitals of carbon atoms overlap with two p orbitals of the oxygen atoms to produce 2 sigma bonds. They are used to form a pi bond as for the two remaining p electrons. In the carbon dioxide molecule, oxygen also hybridizes its orbitals to produce three sp2 hybrid orbitals.
Welcome to a comprehensive exploration of the carbon dioxide molecule or CO2. Lewis structures, also known as electron dot diagrams, were introduced by Gilbert N. Lewis in They play a crucial role in visualizing the arrangement of valence electrons among atoms in a molecule, helping us predict its physical and chemical properties. A Lewis structure is a type of shorthand notation that scientists use to describe the distribution of electrons in molecules. Lewis structures are a foundational tool in chemistry, allowing us to visualize how atoms share or transfer electrons to form molecules.
Hybridization of carbon in co2
First, we need to draw the Lewis structure of CO 2. Write the correct skeletal structure for the molecule. Sum the valence electrons from all the atoms. Use a pair of electrons to form a bond between each pair of bound atoms. Add the remaining electrons to satisfy the octet for a more electronegative atom first. If any atoms lack an octet, make a double or triple bond to give them an octet. The oxygens have 8 electrons. However, the carbon has only four and in these cases, you need to move one of the lone pairs, from the element that has an octet to the element lacking an octet to make another bond:.
Gumtree massage
The absence of the double bonds represents a hybridisation of sp3. What is the bond angle in CO2? Formation of Complexes. But, the carbon has only 4 valence electrons; it does not have octets. Did not receive OTP? This hybridization type occurs as a result of carbon being bound to the other two atoms. What happens next is that one electron from 2s orbital moves from the 2s level to 2p level which results in the formation of two hybrid orbitals. The p orbital in the oxygen atom remains unchanged and is primarily used to form a pi bond. We can see two lone pairs of electrons on each of the oxygen atoms while there is no lone pair of electrons in the carbon atom which means all its valence shell electrons are involved in bonding. Particularly, note down the number of single, double, and triple bonds made by each atom. Allotment of Examination Centre. Zeolites have small, fixed-size openings that allow small molecules to pass through easily but not larger molecules; this is why they are sometimes referred to as molecular sieves. This type of hybridization occurs as a result of carbon being bound to two other atoms. Access free live classes and tests on the app.
We will learn about the hybridization of CO 2 on this page.
It tells various outcomes and results of multiple reactions that add to the hybridisation of carbon dioxide. Lastly, if it is bonded with two atoms, then it is sp. Then, we can complete the octets on the outer shell. However, to form bonds with oxygen, this is not sufficient. It will look like the following. What is the molecular geometry of CO2? This hybridization type occurs as a result of carbon being bound to the other two atoms. It will look as shown below if we started from considering the Oxygen atom. Temporary Hardness of Water. If it is bonded with three atoms, then it is sp2. This shape arises due to the sigma bond and valence electron pairs repelling each other, causing them to move to the opposite side of the carbon atom. And the carbon has 6, which is a bit closer. Does CO2 comprise sp2 hybridisation? The formation of CO 2 consists of two particles: Oxygen and Carbon. The oxygen hybridised its orbital for creating 3 hybrid orbitals of sp2 in the CO2 molecule.

0 thoughts on “Hybridization of carbon in co2”