How many valence electrons does na have
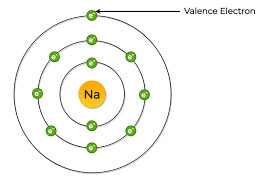
Valence electrons occupy the outermost electron shell in an atom. Sodium, with a total of 11 electrons, has only one electron in its third and outermost shell. Because the outermost shell comes into direct contact with other atoms when a chemical reaction takes place, the valence electrons play a big role in determining the chemical reactivity of an element and the elements with which it will react to form compounds. Elements are arranged in the periodic table according how many valence electrons does na have their valence electrons, with the first group in the first column on the left having a single valence electron.
Valence electrons are the outermost electrons, and are the ones involved in bonding. Sodium has 11 electrons: its atomic number is 11, so it has 11 protons; atoms are neutral, so this means sodium also has 11 electrons. Electrons are arranged in "shells" or energy levels. Depending on your level of Chemistry, it is probably easier to think of them as particles orbiting the nucleus. The first "shell" can have 2 electrons. The second "shell" can have up to 8 electrons.
How many valence electrons does na have
Your tool of choice here will be the Periodic Table of Elements. Grab one and look for sodium , "Na". You'll find it located in period 3 , group 1. The thing to remember about main-group elements , which are those elements located in groups 1 , 2 , and 13 through 18 , is that their group number tells you the number of valence electrons. In your case, sodium is located in group 1 , which means that it has 1 valence electron. This valence electron is located in sodium's third energy shell. How many valence electrons does "Na" have? Stefan V. Aug 4, Sodium has 1 valence electron. Explanation: Your tool of choice here will be the Periodic Table of Elements. Related questions How do valence electrons affect chemical bonding? How do valence electrons determine chemical properties? How do valence electrons determine chemical reactivity? How many valence electrons are in a silicon atom?
What is a Polyatomic Ion?
The electronic configuration is 2 , 8. Therefore, the number of valence electrons in sodium ion is 8. The electronic configuration is 2, 8. Therefore, the number of valence electrons in O 2- is 8. Menu Categories.
Sodium, a chemical element with symbol Na, is one of the highly reactive alkali metals of group 1 with atomic number 11 in the periodic table. Sodium is not found in a free state in nature due to its high reactivity behavior so that it is abstracted from different compounds mostly from salts. But for this you have to know what these two terms are, so without wasting your time let's go for it,. Valence electrons are the total number of electrons present in the outermost shell of an atom i. The valence electrons for a neutral atom is always definite, it cannot be varied more or less in any condition for a particular atom and may or not be equal to its valency. There are four simple steps to find out the valence electrons for sodium atom which are:.
How many valence electrons does na have
If you're seeing this message, it means we're having trouble loading external resources on our website. To log in and use all the features of Khan Academy, please enable JavaScript in your browser. Search for courses, skills, and videos. The periodic table and Lewis diagrams. Learn how to determine the number of valence electrons for an element using the periodic table. An atom's valence electrons are the electrons in its outermost shell. A Bohr model of a chlorine atom shows a nucleus surrounded by three concentric rings. The ring closest to the nucleus contains 2 black dots, the second ring from the nucleus contains 8 black dots, and the third ring from the nucleus contains 7 red dots.
O2tvseries
In solution, the sodium and chlorine atoms separate to form sodium and chlorine ions, but the sodium valence electron stays with the chlorine atom. How do valence electrons determine chemical reactivity? How many valence electrons does "Na" have? This trend only gets broken at Sc You'll find it located in period 3 , group 1. You can reuse this answer Creative Commons License. Valence electrons are found in the highest energy s and p orbitals in the main group of elements. For sodium, which is in the 3rd row of the periodic table , the valence electrons will be found in the 3rd shell. Quick note: In the periodic table, rows are horizontal lines, rows are vertical lines. The two opposite charges attract, and the two atoms now form the molecule of a compound. What is the reason for positive charge on a sodium ion and a negative charge on a chloride ion? When the sodium reacted with the chlorine to form sodium chloride, the single sodium valence electron jumped over to fill the hole in chlorine's valence electron shell. Impact of this question views around the world. The transfer of the electron results in a chemical bond and the formation of a compound.
You may assume that the valences of the elements—the number of electrons with which an atom will bond or form—are those that can be derived by looking at the groups columns of the periodic table.
The electronic configuration is 2 , 8. Valence electrons are electrons found in the outermost shell of an atom. Na, K, Rb, Cs, Fr. How do valence electrons determine chemical properties? The transfer of the electron results in a chemical bond and the formation of a compound. Therefore, the number of valence electrons in O 2- is 8. The solution is stable, the ions with their complete outer shells not engaging in any further chemical reactions. How do you calculate number of valence electrons? The number of electrons in its ion E2— will be : a 16 b 18 c 15 d Sodium and chlorine react strongly to form sodium chloride or table salt, a stable compound.

Without variants....