How many electrons are in chlorine
Examples of Compounds with Chlorine Sodium Chloride This is sodium chloridealso known as table salt. Most people scientist know that the formula for salt is NaCl. One sodium Na atom gives it's electron to one chlorine Cl atom. Chlorine then has the eight electrons in its outer shell to make it "happy".
Additionally, a more basic way of determining the number of valence electrons would be to simply look at what group Cl is in. Chlorine has an atomic number Atomic number is the number of protons present in the nucleus of an atom. All the atoms of an element have same atomic number. In every stable atom the number of electrons is equal to the number of protons.
How many electrons are in chlorine
Electron Configuration Notation: -shows the arrangment of electrons around the nucleus of an atom. How to Write the Electron Configuration for Chlorine Cl In order to write the Chlorine electron configuration we first need to know the number of electrons for the Cl atom there are 17 electrons. When we write the configuration we'll put all 17 electrons in orbitals around the nucleus of the Chlorine atom. In writing the electron configuration for Chlorine the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons the next 2 electrons for Chlorine go in the 2s orbital. The next six electrons will go in the 2p orbital. The p orbital can hold up to six electrons. We'll put six in the 2p orbital and then put the next two electrons in the 3s. Since the 3s if now full we'll move to the 3p where we'll place the remaining five electrons. Therefore the Chlorine electron configuration will be 1s 2 2s 2 2p 6 3s 2 3p 5.
When we write the configuration we'll put all 17 electrons in orbitals around the nucleus of the Chlorine atom. In writing the electron configuration for Chlorine the first two electrons will go in the 1s orbital. Chlorine has an atomic number
Discover all the products made possible by chlorine chemistry through our chlorine and sodium hydroxide product trees. Chlorine Facts and Science. Chlorine is an element with unique properties Elemental chlorine gas Cl 2 is a yellow-green gas at room temperature and has a pungent odor similar to bleach even at very low concentrations. Chlorine has an atomic number of 17 and an atomic mass of As a member of the halogen family on the Periodic Table, chlorine is very reactive with metals and forms salts. The density of chlorine is The high density of chlorine gas causes it to sink if released into the ambient environment.
Although we have discussed the general arrangement of subatomic particles in atoms, we have said little about how electrons occupy the space about the nucleus. Do they move around the nucleus at random, or do they exist in some ordered arrangement? The modern theory of electron behavior is called quantum mechanics. It makes the following statements about electrons in atoms:. It is the arrangement of electrons into shells and subshells that most concerns us here, so we will focus on that. We use numbers to indicate which shell an electron is in. As shown in Table 2. This first shell has only one subshell, which is labeled 1 s and can hold a maximum of 2 electrons.
How many electrons are in chlorine
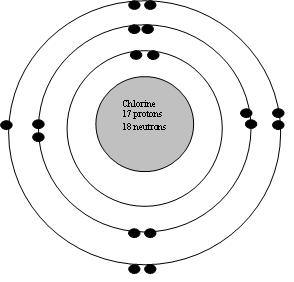
In fact, the table mentioned below is the perfect information box Which gives you every single detail about the Chlorine element in Periodic table. See how this Interactive Periodic Table helps you. Chlorine element is in group 17 and period 3 of the Periodic table. Chlorine is the p-block element and it belongs to halogens group. Click on above elements in Periodic table to see their information or Visit Interactive Periodic Table which shows names, symbol, atomic mass, electron configuration, electrons arrangement, etc. Click on above elements in Periodic table to see their information. Do you know, how many electrons can be accommodated in the first shell, second shell, third shell, fourth shell, etc…? This electron arrangement indicates that the outermost orbit of chlorine element Cl has 7 electrons. From the Bohr model, it can be found that the number of orbits or shells in chlorine is 3. Hence, as chlorine has 3 orbits, it lies in period 3 of the Periodic table.
Memes fimosis
Here is a video which gives a quick discussion of how to determine how many valence electrons atoms of different elements have. Here, stable means that atom has not formed a ion yet. How many valence electrons are in an atom of chlorine? Magnesium chloride MgCl 2 —Found in seawater and serves as a natural source of metal magnesium. Stable means that atom has not formed a ion yet. Therefore, there are 7 valence electrons present in an chlorine atom. Current Page: Chem4Kids. Overall, chlorine is the 23 rd most prevalent element in the universe. Some volcanoes emit elemental chlorine gas Cl 2. This means, that the number of electrons present in an chlorine atom is The electronic configuration of chlorine atom is:- E.
Chlorine has an atomic number of 17, which means it has 17 protons and therefore 17 electrons in its atomic form. We'll need to know how many sublevel is present in each energy level, and in turn, how many electrons each sublevel can accommodate. From the given table, for energy level 1, there's only 1 sublevel, which is called 1s.
Drinking water—A major part of the water treatment process, chlorine-based disinfectants have residual disinfection activity that prevents the regrowth of pathogens in the water distribution system. Chem4Kids Sections. This means, that the number of electrons present in an chlorine atom is Some volcanoes emit elemental chlorine gas Cl 2. Stable means that atom has not formed a ion yet. Hydrochloric acid also has many uses including processing steel and food products like gelatin and sugar, and producing batteries. Jan 6, How many valence electrons are in an atom of bromine? Because aluminum has three, that means three chlorine atoms can bond. Each of the chlorine atoms gets an electron to fill its shell, and the aluminum loses three, giving it a filled shell too remember, Aluminum has three extra electrons. One sodium Na atom gives it's electron to one chlorine Cl atom. How many valence electrons are in an atom of chlorine? Now, the last shell of chlorine atom has 7 electrons in it. Therefore, there are 7 valence electrons present in an chlorine atom. Nitrogen shares its electrons with the chlorine atoms, so all of the atoms have their shells filled.

And where logic?
I apologise, but, in my opinion, you are not right. I am assured. I can prove it.