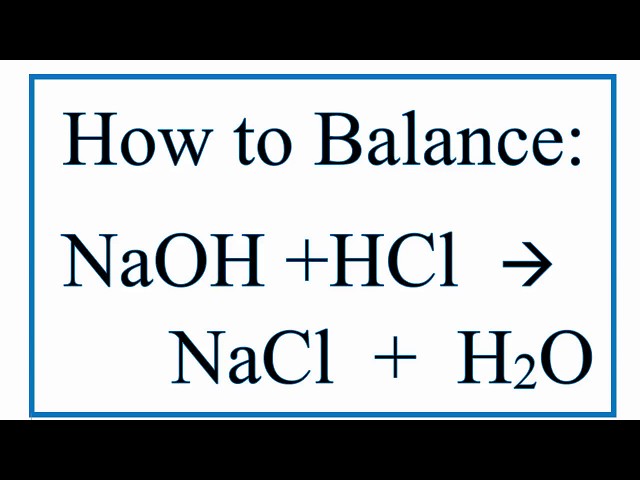
Hcl plus naoh
Key Points. Additional Information.
Hint: This graph cannot be done by Excel. Select all that apply. NaOH is a strong base and HCl is a strong acid. Thus, the pH of the resultant solution will be determined based on the amount of excess reagent. When doing pH calculations for titrations, the most commonly made mistake is forgetting to take into account the change in volume.
Hcl plus naoh
Last updated on Dec 30, HTET Notificatoon to be out soon! Candidates applied online from 30th October to 11th November This is a great opportunity for teaching job aspirants. Get Started. SSC Exams. Banking Exams. Teaching Exams. Civil Services Exam. Railways Exams. Engineering Recruitment Exams. Defence Exams. State Govt. Police Exams.
EMRS Librarian. Gujarat Police ASI. JNU Junior Assistant.
The thing to recognize here is the fact that you're dealing with a neutralization reaction that features sodium hydroxide, "NaOH" , a strong base , and hydrochloric acid, "HCl" , a strong acid. This tells you that the two reactants will dissociate completely in aqueous solution to produce cations and anions. More specifically, you will have. Now, when these two solutions are mixed, the hydroxide anions produced by the strong base and the hydrogen ions produced by the strong acid will neutralize each other to produce water. The sodium cations and the chloride anions act as spectator ions because they are present on both sides of the chemical equation as ions. You can tell that this is the case because sodium chloride, "NaCl" , one of the two products of the reaction, is soluble in aqueous solution. In this case, the dissociation of hydrochloric acid is represented by the chemical equation.
Direct link to this balanced equation:. A chemical equation represents a chemical reaction. It shows the reactants substances that start a reaction and products substances formed by the reaction. However, this equation isn't balanced because the number of atoms for each element is not the same on both sides of the equation. A balanced equation obeys the Law of Conservation of Mass, which states that matter is neither created nor destroyed in a chemical reaction.
Hcl plus naoh
As we have seen in the section on chemical reactions, when an acid and base are mixed, they undergo a neutralization reaction. This is sometimes true, but the salts that are formed in these reactions may have acidic or basic properties of their own, as we shall now see. A solution is neutral when it contains equal concentrations of hydronium and hydroxide ions. When we mix solutions of an acid and a base, an acid-base neutralization reaction occurs. However, even if we mix stoichiometrically equivalent quantities, we may find that the resulting solution is not neutral. It could contain either an excess of hydronium ions or an excess of hydroxide ions because the nature of the salt formed determines whether the solution is acidic, neutral, or basic. The following four situations illustrate how solutions with various pH values can arise following a neutralization reaction using stoichiometrically equivalent quantities:. Our stomachs contain a solution of roughly 0. The burning sensation associated with heartburn is a result of the acid of the stomach leaking through the muscular valve at the top of the stomach into the lower reaches of the esophagus. The lining of the esophagus is not protected from the corrosive effects of stomach acid the way the lining of the stomach is, and the results can be very painful.
Sgcib
Haryana Police. All Rights Reserved. Patna High Court Assistant. The homolytic fission of a covalent bond liberates :. Civil Services Exam. When phenophthalein is added to NaOH solution ,the solution becomes pink. Rajasthan GNM. The disease caused by breathing polluted air is:. This colour change occurs abruptly because. Bihar Panchayati Raj Clerk. Rajasthan High Court. AP Police SI. NaOH is a strong base and HCl is a strong acid. What are the reactants in this reaction naoh hcl -- nacl h2o?
If you're seeing this message, it means we're having trouble loading external resources on our website.
Which of these waves is a Electromagnetic waves? India Post Mail Guard. MP Cooperative Bank Clerk. Assam Animal Husbandry Veterinary. Accounting and Commerce. How many images will be formed if angle between mirrors is 30 degree? Question 19eb9. Insurance Exams. West Bengal Group D. TN Forest Guard.

Bravo, what phrase..., a remarkable idea