Hbr lewis structure
HBr hydrogen bromide lewis structure has one Hydrogen atom H and one Bromine atom Br which contain a single bond between them. There are 3 lone pairs on the Bromine atom Br.
HBr is the chemical formula of hydrogen bromide. We are learning here about HBr lewis structure, characterizations and quick facts. Hydrogen bromide is an anhydrous gas with no colour having strong irritating smell. It is corrosive in nature and heavier than air. HBr molecule contains one hydrogen atom and one bromine atom in its structure. The molecular weight of HBr is
Hbr lewis structure
There are no charges on atoms in HBr lewis structure because HBr is a neutral molecule. There is three lone pairs on bromine atom in HBr molecule. HBr is a very easy lewis structure to draw due to its simplicity. There are only one hydrogen atom and one bromine atom in HBr molecule. In the lewis structure of HBr, hydrogen atom has made a single bond with bromine atom. When we draw a lewis structure, there are several steps to follow. Number of steps can be changed according the complexity of the molecule or ion. Because HBr molecule is a simple molecule and there is no overall charge, all of these steps are not required to use to complete the lewis structure. However those all steps are mentioned and explained in detail in this tutorial for your knowledge. There are two elements in hydrogen bromide; hydrogen and bromine. Hydrogen is a group IA element in the periodic table and only has one electron in its last shell valence shell. Bromine is a group VIIA element in the periodic table and contains seven electrons in its last shell. Now, we know how many electrons are there in valence shells of hydrogen and bromine atoms. Total electron pairs are determined by dividing the number total valence electrons by two. For, HBr, Total pairs of electrons are four in their valence shells.
Why HBr soluble in water?
.
In all cases, these bonds involve the sharing or transfer of valence shell electrons between atoms. In this section, we will explore the typical method for depicting valence shell electrons and chemical bonds, namely Lewis symbols and Lewis structures. We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons:. Figure 1 shows the Lewis symbols for the elements of the third period of the periodic table.
Hbr lewis structure
HBr hydrogen bromide has one hydrogen atom and one bromine atom. In the HBr Lewis structure, there is a single bond between the hydrogen and bromine atom, and on the bromine atom, there are three lone pairs. In the periodic table , hydrogen lies in group 1, and bromine lies in group Hence, hydrogen has one valence electron and bromine has seven valence electrons. Learn how to find: Hydrogen valence electrons and Bromine valence electrons. We have a total of 8 valence electrons. And when we divide this value by two, we get the value of total electron pairs. Here hydrogen can not be the central atom.
Synonimy
Hence, HBr is an inorganic compound. Why HBr has higher boiling point? Yes, HBr is a volatile acid. Because, there are only two elements in HBr molecule, we do not need to worry about finding the center atom. Is HBr conductive? HBr molecule contains one hydrogen atom and one bromine atom in its structure. No, HBr is not a nucleophile. So, the HBr molecule has linear molecular shape and tetrahedral electron geometry as it has unequal distribution of electrons in HBr. There is three lone pairs on bromine atom in HBr molecule. HBr is the chemical formula of hydrogen bromide. Why HBr is polar?
Chapter 1 Chapter 1: The Chemical World 1.
HBr hydrobromic acid cannot show both acidic and basic nature. Strong electrolytes are the compounds which get completely ionized into water to conduct electricity. Now in the HBr molecule, you have to put the electron pairs between the hydrogen atom H and bromine atom Br. Remember that, there are total of four electron pairs. Hence, total electron pairs of HBr lewis structure is four. Yes, HBr is present in both a gas and a liquid. He is a founder of Pediabay and is passionate about helping students through his easily digestible explanations. HBr hydrogen bromide or hydrobromic acid is an inorganic compound. In the above lewis dot structure of HBr, you can also represent each bonding electron pair : as a single bond. By doing so, you will get the following lewis structure of HBr. Thus it creates partial positive charge on H atom and partial negative charge on Br atom. There are only one hydrogen atom and one bromine atom in HBr molecule. The HBr molecule has a total 8 valence electrons and out of these, only 2 valence electrons are used in the above sketch.

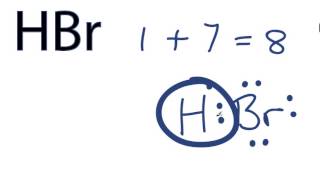
0 thoughts on “Hbr lewis structure”