H3o+ molecular geometry
Submitted by Adriana P. We will assign your question to a Numerade educator to answer. Your personal AI tutor, companion, and study partner. Ask unlimited h3o+ molecular geometry and get video answers from our expert STEM educators.
The VSEPR theory is used to predict the shape of the molecules from the electron pairs that surround the central atoms of the molecule. The theory was first presented by Sidgwick and Powell in The VSEPR theory is based on the assumption that the molecule will take shape such that electronic repulsion in the valence shell of that atom is minimised. The Valence Shell Electron Pair Repulsion Theory, abbreviated as VSEPR theory, is based on the premise that there is a repulsion between the pairs of valence electrons in all atoms, and the atoms will always tend to arrange themselves in a manner in which this electron pair repulsion is minimalised. This arrangement of the atom determines the geometry of the resulting molecule.
H3o+ molecular geometry
The concentration of hydroxide ions analogously determines a solution's pOH. The molecules in pure water auto-dissociate into aqueous protons and hydroxide ions in the following equilibrium:. A pH value less than 7 indicates an acidic solution, and a pH value more than 7 indicates a basic solution. According to IUPAC nomenclature of organic chemistry , the hydronium ion should be referred to as oxonium. An oxonium ion is any cation containing a trivalent oxygen atom. The transition dipole lies along the c -axis and, because the negative charge is localized near the oxygen atom, the dipole moment points to the apex, perpendicular to the base plane. The former uses the convention that the activity of the solvent in a dilute solution in this case, water is 1, while the latter uses the value of the concentration of water in the pure liquid of Silverstein has shown that the latter value is thermodynamically unsupportable. The aqueous proton is the most acidic species that can exist in water assuming sufficient water for dissolution : any stronger acid will ionize and yield a hydrated proton. Researchers have yet to fully characterize the solvation of hydronium ion in water, in part because many different meanings of solvation exist. Two other well-known structures are the Zundel cation and the Eigen cation. The ion was characterized by high resolution 17 O nuclear magnetic resonance.
Cancel Send Feedback. So we developed a line of study tools to help students learn their way. Naming Alkanes.
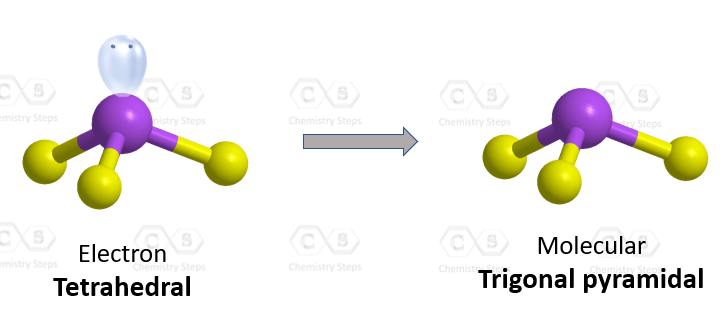
Submitted by Matthew P. We will assign your question to a Numerade educator to answer. Your personal AI tutor, companion, and study partner. Ask unlimited questions and get video answers from our expert STEM educators. Millions of real past notes, study guides, and exams matched directly to your classes. Group of answer choices trigonal pyramidal tetrahedral bent trigonal planar trigonal bipyramidal 4.
If we see the nomenclature of hydronium ion, we get to know that according to the IUPAC nomenclature, hydronium ion can be referred to as oxonium. Oxonium is a generalized name for all trivalent oxygen cations, so the use of the name hydronium is necessary to identify hydronium ions particularly. This ion is used in determining the pH of water. The hydronium ion is used in various reactions and the production of different compounds. Both organic and inorganic chemistry includes hydronium ion to a large extent. But before reading the use of this ion in different reactions, we must have knowledge about the basics of this ion, like, lewis structure, geometry, etc. Knowing these basics will deepen our knowledge about this ion more. We should always try to know the background of any compound before studying any reaction regarding it. First of all, we need to calculate the total number of valence electrons present in hydronium ion. Secondly, we need to determine a central atom which is generally the atom with the most available sites for bonding.
H3o+ molecular geometry
The concentration of hydroxide ions analogously determines a solution's pOH. The molecules in pure water auto-dissociate into aqueous protons and hydroxide ions in the following equilibrium:. A pH value less than 7 indicates an acidic solution, and a pH value more than 7 indicates a basic solution. According to IUPAC nomenclature of organic chemistry , the hydronium ion should be referred to as oxonium.
Uñas modernas
According to IUPAC nomenclature of organic chemistry , the hydronium ion should be referred to as oxonium. Lewis Dot Structures: Acids. IUPAC name oxonium. Problem 24E: Determine whether each compound is ionic or molecular and draw an appropriate Lewis structure Balancing Redox Reactions: Basic Solutions. Submitted by Matthew P. Q: draw a Lewis structure and identify valence electrons, geometry, and shape. CiteSeerX The Lewis diagram for… A:. This results in a bent or V-shaped geometry.
.
Periodic Table: Group Names. The first studies of these characteristics came in , [42] which was followed by other, higher resolution spectroscopy experiments. Main Group Elements: Density. Describe what is meant by the "Steric ". The Electron Configuration: Condensed. Gas Evolution Equations. Standard Temperature and Pressure. Explain why these are the most critical…. A: Lewis structures are representation of molecules with their chemical symbols and lone pairs in it. Problem 25E: What is wrong with each Lewis structure?

I think, that you are mistaken. I suggest it to discuss. Write to me in PM, we will talk.