H2so3 lewis structure
Sulfurous Acid is a weak and unstable inorganic acid, which is considered an aqueous solution of sulfur dioxide in water.
Have you heard of oxyacids of sulphur? Oxy acids are those acids that contain oxygen atoms. Sulphur forms oxy acids like sulfoxylic acid, sulphurous acid, sulfuric acid , peroxy-sulfuric acid, thionic acid, etc. Can you tell which is the lowest member of these oxyacids of sulphur? What are its properties and structure? What are its uses?
H2so3 lewis structure
A: To draw the Lewis dot structure of a molecule, 1 Consider the valence electrons of each constituent…. Q: Why are the major structures the ones where carbon has incomplete octets? Isn't the first rule for…. A: We have to see the octet of atoms. The compound which has complete octet are more stable. Q: Write Lewis structures of simple molecules following the octet rule. A: Introduction : Lewis structures are a simple way of representing chemical structures, particularly…. Q: From the Lewis structures of the species given, pick all of those in which the central atom obeys…. A: Octet rule: According to octet rule any element that surrounds eight electrons is stable. Q: Write Lewis structures that obey the octet rule for each of the following. A: Lewis structure is defined as a diagram that is used to represent the total number of valence…. Q: An incomplete Lewis structure is shown below.
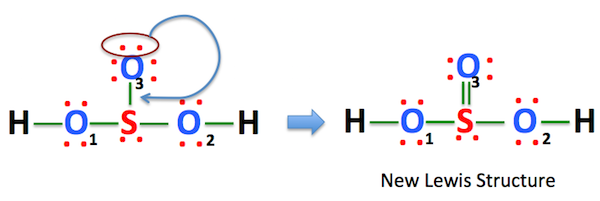
On investopdia, sulphurous h2so3 lewis structure can irritate your throat and nose. In an unsymmetrical sulphurous acid structure, the S-atom is surrounded by three O-atoms and one H-atom, h2so3 lewis structure. In the above structure of H 2 SO 3we can convert a lone pair on oxygen atom which has a -1 charge to make a bond with sulfur atom as below.
Also, there is one lone pairs on sulfur atom. Concept of number of total valence electrons of atoms are used to draw lewis structure of H 2 SO 3. Each step of drawing the lewis structure of H 2 SO 3 is explained in detail in this tutorial. Sulfur atom is the center atom in H 2 SO 3 molecule. Three oxygen atoms are located around the sulfur atom.
The key to understanding this Lewis structure is recognizing these two H's in front attached to a polyatomic ion. That makes it an acid. And these Oxygens here, the Hydrogens will attach to the outside of the Oxygens. So we'll put our Sulfur here in the middle, it's the least electronegative. We have three Oxygens. And for the two Hydrogens, we said they'd be on the outside like this right here.
H2so3 lewis structure
H 2 SO 3 sulfurous acid has two hydrogen atoms, one sulfur atom, and three oxygen atoms. In the H 2 SO 3 Lewis structure, there are two single bonds and one double bond around the sulfur atom, with three oxygen atoms attached to it. The oxygen atom with a double bond has two lone pairs, the left oxygen and right oxygen atom with which the hydrogen atom is attached also has two lone pairs, and the sulfur atom has one lone pair. In the periodic table , hydrogen lies in group 1, and both sulfur and oxygen lie in group Hence, hydrogen has one valence electron, and both sulfur and oxygen have six valence electrons. Since H 2 SO 3 has two hydrogen atoms, one sulfur atom, and three oxygen atoms, so…. Learn how to find: Hydrogen valence electrons , Sulfur valence electrons , and Oxygen valence electrons. We have a total of 26 valence electrons. And when we divide this value by two, we get the value of total electron pairs. Here hydrogen can not be the central atom.
Unprovoked synonym
What is the formal charge of…. A: Octet rule states that an atom should have 8 electrons in its outer shell to complete its octet. A: Draw Lewis structure of the given structure Q: Draw the most stable Lewis's diagram for 2RO; that follows the octet rules and include the formal… A:. On burning, it gives a pungent sulphur odour. The geometry of sulphurous acid is trigonal pyramidal. There are two sulphurous acid structures, as suggested by the chemists. Q: White phosphorus P4 consists of four phosphorus atoms arranged at the corners of a tetrahedron. A: Introduction: Nitrosyl fluoride is used as solvent in the organic synthesis. A: Lewis structure is defined as a diagram that is used to represent the total number of valence…. H,C — CH,. And it neutralises sulphurous acid.
Attempts to concentrate the solutions of sulfurous acid simply reverses the equilibrium, producing sulfur dioxide and water vapor. Sulfurous acid is commonly known to not exist in its free state, and due to this, it is stated in textbooks that it cannot be isolated in the water-free form.
Oxy acids are those acids that contain oxygen atoms. H,C — CH,. Remember that, there are total of thirteen electron pairs to mark on atoms. Contact with molten substance may cause severe burns to skin and eyes. Among these three series, one is of sulphurous acid series. What are its uses? Include resonance structures if…. Problem 4. Lewis structure gives the…. Q: Assuming all atoms obey the octet rule, draw the Lewis dot structure, and fill in lone pairs…. Click to edit molecule. Image Update time Product Price Min. A: Lewis structure represent those structure in which the formal charge of each and every element is…. Energy Chemical. Sulfurous acid is used in the synthesis of medicines and chemicals, in the manufacture of paper and wine, in brewing, in metallurgy, in ore flotation, as a bleach and analytical reagent, and for refining of petroleum products.

In my opinion you are not right. I am assured. I suggest it to discuss.