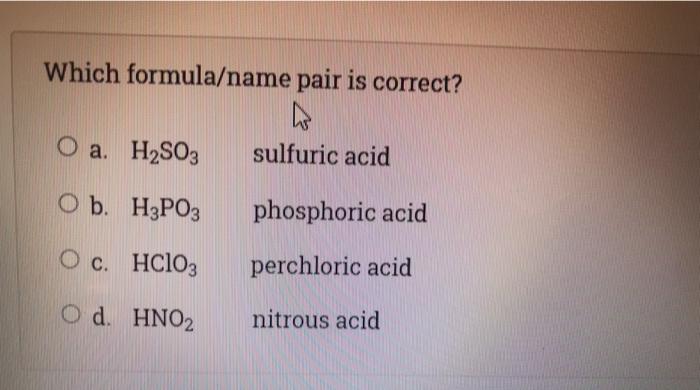
H2so3 compound name
It is a colorless, viscous liquid that is slightly soluble in water. It is a strong acid that can dissolve many metals. Verify OTP Code required.
Route of exposure:. Biological location:. Organ and components:. Biofluid and excreta:. Indirect biological role:. Biological role:.
H2so3 compound name
Wiki User. Sulfurous Acid. And for H2SO4 it is hydrogen Sulphate. Sulphurous acid. Compounds are made of two or more kinds of elements combined chemicaly. So a compound plus a compound cannot be combined because a compound is combined elements. H2O can react with another compound eg. H2SO3 is sulfurous acid. Sulphurous acid is not a reaction chemical or physical ; the sulphurous acid H2SO3 is a chemical compound. H2SO3 is the chemical formula of the sulfurous acid.
Sulfurous acid and its salts are commonly applied as powerful reducing agents and disinfectant agents due to its strong reducibility. HH
Sulfurous Acid is a weak and unstable inorganic acid, which is considered an aqueous solution of sulfur dioxide in water. It is formed theoretically by burning sulfur to produce sulfur dioxide, which is then reacted with water. However, there is no evidence that sulfurous acid exists in solution, while the molecules of which has been detected in the gas phase, since the reaction is reversible and the acid readily decomposes back into the reactants. Sulfurous acid is not usually available in its acid form, but more commonly prepared as its sodium or potassium salts. Sulfurous acid and its salts are commonly applied as powerful reducing agents and disinfectant agents due to its strong reducibility. It is also considered as a mild bleaching agent especially for applications having chlorine sensitive materials. Sulfurous acid H2SO3 can be produced by burning sulfur to form sulfur dioxide SO2 gas and by then dissolving the gas in water to form sulfurous acid.
Sulfurous Acid is a chemical compound which has a formula H 2 SO 3 , and is a weak and unstable acid, formed when sulfur dioxide dissolves in water. The facts that sulfur dioxide actually exists in solution, cannot be said surely, but the molecules of this substance has been detected in the gas phase. It is a reducing, as well as a bleaching agent. The sulfurous acid compound is only formed in the aqueous solution, and is therefore not isolated in its pure state. This is the Sulfurous Acid Chemical formula, since it atoms of hydrogen, oxygen and carbon are joined by a strong chemical bond. This is also the molecular formula for sulfurous acid, since it demonstrates that one molecule of sulfurous acid contains two atoms of hydrogen, one atom of sulfur and three atoms of oxygen.
H2so3 compound name
A spot test for gold has been in use for decades. The sample is first treated with nitric acid. Other metals may react or dissolve in this acid, but gold will not. Then the sample is added to a mixture of nitric acid and hydrochloric acid.
Pegging amateur porn
Acidic nature: It shows dibasic nature. Frequently Asked Questions 1. On inhalation, sulphurous acid can irritate your throat and nose. It can even cause damage to your eyes. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students. The acid has a sour taste and is corrosive. ICSC TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Examples of molecular weight computations: C[14]O[16]2 , S[34]O[16]2. Sulfurous Acid price More Price 7. The geometry of sulphurous acid is trigonal pyramidal. Schwarz Toxicity of Sulphurous Acid Apart from sulphurous acid uses, there are some toxicity issues. Although handling of SO 2 liquid requires special equipment, it is frequently used as a pH regulator and depressant, primarily during the treatment of complex sulfide ores.
A spot test for gold has been in use for decades. The sample is first treated with nitric acid. Other metals may react or dissolve in this acid, but gold will not.
GHS labelling :. In adverse cases,. Sulfurous Acid is a weak and unstable inorganic acid, which is considered an aqueous solution of sulfur dioxide in water. Does sulphurous acid cause health hazards? Property Value Source logP. A corrosive irritant to skin, eyes, and mucous membranes. These are Sulphurous acid is a strong reducing agent. Tools Tools. What is the compound formed when sulfur dioxide reacts with moisture in clouds? What are its uses? Chemistry tools. It is a strong acid that can dissolve many metals. A dibasic acid, that is. Related compounds. Normally, both sulphurous acid structures exist in resonance with each other.

I think, that you are not right. I am assured. Let's discuss.
I think, that you are mistaken. I suggest it to discuss. Write to me in PM, we will talk.
You are not right. Let's discuss. Write to me in PM.