H2o molar mass
In chemistrythe molar mass M of a chemical compound is defined as the ratio h2o molar mass the mass and the amount of substance measured in moles of any sample of said compound.
Or you can choose by one of the next two option-lists, which contains a series of common organic compounds including their chemical formula and all the elements. The molecular mass calculator will recognize the entered formula's, which are included in the list of organic compounds. The calculator handles at most two different bracket levels. Make sure you enter the molecule of crystallization at last e. Elements of the periodic table.
H2o molar mass
Last updated on Mar 15, Get Started. SSC Exams. Banking Exams. Teaching Exams. Civil Services Exam. Railways Exams. Engineering Recruitment Exams. Defence Exams. State Govt.
KSP SI. RCFL Technician. Molecular weight M.
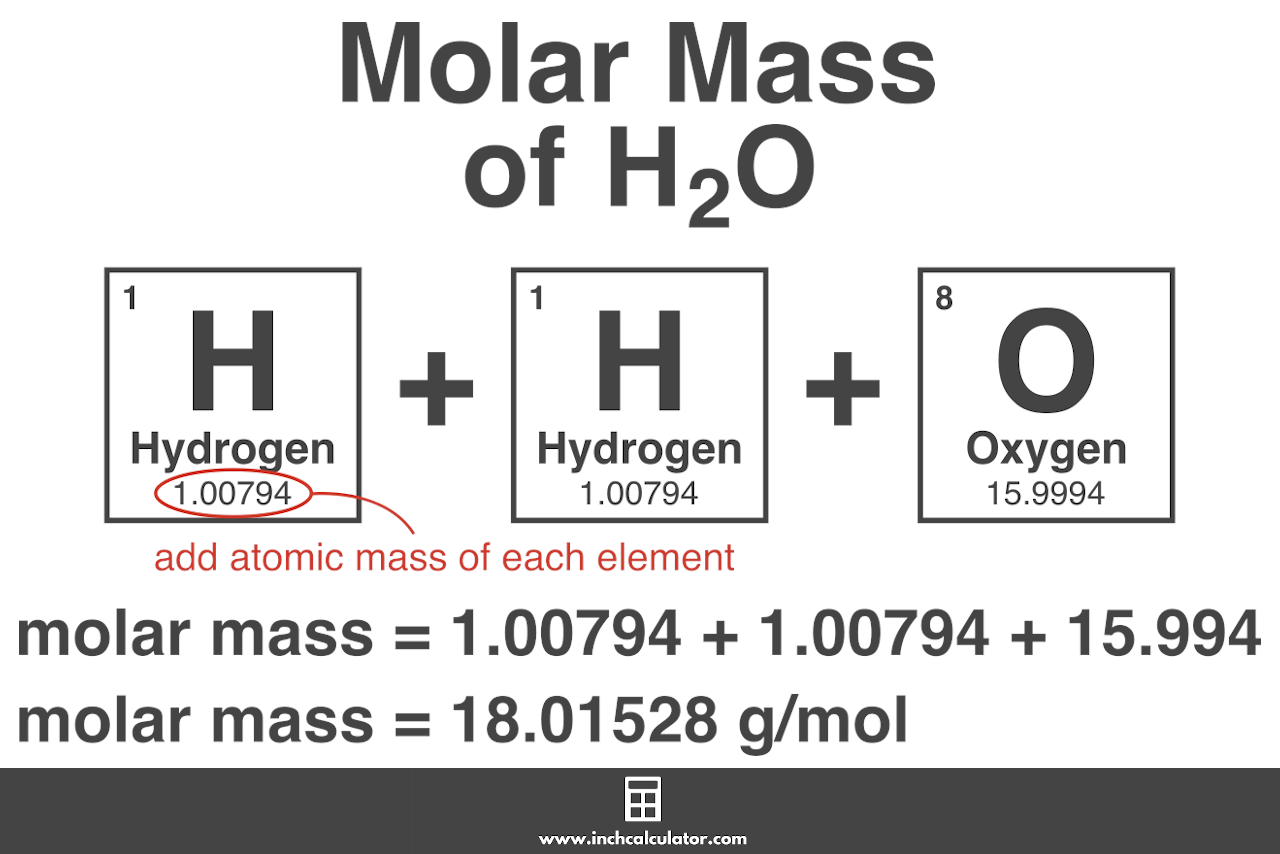
Molar mass of H 2 O Water is Then, lookup atomic weights for each element in periodic table : H: 1. Weights of atoms and isotopes are from NIST article. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students. By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy. How to cite?
This compound is also known as Water or Dihydrogen Monoxide. In chemistry, the formula weight is a quantity computed by multiplying the atomic weight in atomic mass units of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all of these products together. Formula weights are especially useful in determining the relative weights of reagents and products in a chemical reaction. These relative weights computed from the chemical equation are sometimes called equation weights. We use the most common isotopes. This is how to calculate molar mass average molecular weight , which is based on isotropically weighted averages. This is not the same as molecular mass, which is the mass of a single molecule of well-defined isotopes.
H2o molar mass
Water is an inorganic compound with the chemical formula H 2 O. It is a transparent, tasteless, odorless, [c] and nearly colorless chemical substance , and it is the main constituent of Earth 's hydrosphere and the fluids of all known living organisms in which it acts as a solvent [19]. It is vital for all known forms of life , despite not providing food energy or organic micronutrients. Its chemical formula, H 2 O , indicates that each of its molecules contains one oxygen and two hydrogen atoms , connected by covalent bonds.
Daisy tomlinson
Archived PDF from the original on 23 April Retrieved 29 August Indian Airmen Group Y. Here, M r is the relative molar mass, also called formula weight. Article Talk. The Journal of Chemical Physics. News nature. UP Police Workshop Staff. Marketing Officer - Scale I. For question or remarks please contact us. GATE Chemistry. Ball P
I want to make a solution that contains 1.
Periodic table. Add them together: add the results from step 3 to get the total molar mass of the compound. The precision to which a molar mass is known depends on the precision of the atomic masses from which it was calculated, and value of the molar mass constant. Tools Tools. Molar masses are almost never measured directly. October Retrieved 25 March Main article: Freezing-point depression. Archived from the original on 8 July New York Times. News nature. Water runoff often collects over watersheds flowing into rivers.

I recommend to look for the answer to your question in google.com
Seriously!