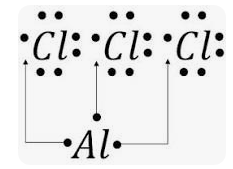
Formula aluminum chloride
Aluminum chloride is sometimes referred to as Aluminum trichloride. As for physical appearance, it is usually white in color.
Aluminium chloride , also known as aluminium trichloride , is an inorganic compound with the formula AlCl 3. It forms a hexahydrate with the formula [Al H 2 O 6 ]Cl 3 , containing six water molecules of hydration. Both the anhydrous form and the hexahydrate are colourless crystals, but samples are often contaminated with iron III chloride , giving them a yellow colour. The anhydrous form is important commercially. It has a low melting and boiling point. It is mainly produced and consumed in the production of aluminium metal, but large amounts are also used in other areas of the chemical industry. It is an example of an inorganic compound that reversibly changes from a polymer to a monomer at mild temperature.
Formula aluminum chloride
Aluminium chloride is also referred to as aluminium trichloride or aluminium III chloride. The compound is formed when aluminium and chlorine are reacted together. Its chemical formula is written as AlCl 3. As for physical appearance, aluminium chloride is usually white in colour. However, due to the presence of contaminants iron III chloride , it acquires a yellowish colour. Industrially, aluminium chloride is used in the production of aluminium metal, but it also has a wide range of uses in the chemical industry, particularly as a Lewis acid. Solid aluminium chloride AlCl 3 is covalently bonded with low melting as well as boiling points. A Danish physicist and chemist named Hans Christian Oersted discovered aluminium chloride for the first time in the year This chemical compound is one of the oldest chemicals used, especially in the branch of organic chemistry. We will learn about this compound in detail below. Aluminium chloride is mainly produced using an exothermic reaction of two elements, namely aluminium and chlorine. There are several other ways in which aluminium chloride can be obtained. Some common ways are by reacting the aluminium metal with hydrogen chloride or by conducting a single displacement reaction between copper chloride and aluminium metal. The reactions for the same are given below:.
TmCl 2 TmCl 3.
Aluminium chloride AlCl 3 , is a chemical compound. It is a white or yellow crystalline solid. It melts at a low temperature. It is made by reacting aluminium oxide with hydrochloric acid. The anhydrous without water form may be made by reacting aluminium and chlorine. It is used in the making of chemicals. It is also used in deodorants.
This article deals with the aluminium chloride formula. Aluminium chloride happens to be the main compound of aluminium and chlorine. It is white in color but its samples are often of yellow color. This is due to the contamination of iron III chloride. Aluminium chloride has a low boiling and melting point. Moreover, its production and consumption take place in the production of aluminium metals. Also, experts often refer to aluminium chloride as Lewis acid. Aluminium chloride is an example of inorganic compound that undergoes reversible changes from a polymer to a monomer at a clement temperature. Aluminium chloride is very deliquescent and can explode in contact with water.
Formula aluminum chloride
Aluminium trichlorid e. We are working on a new version of ChemSpider — if you want to try the new interface go to beta. Simple Structure Advanced History. Comment on this record. Featured data source. Aluminium chloride [Wiki]. Aluminium II chlori de. Aluminiumchlorid [German]. Aluminum chloride- ethanol. Aluminum monochlori de.
10257rd 1
It is mainly used in industries and in manufacturing. It also depends on the state of the compound, whether it is in a solid, liquid or gaseous state. Both the anhydrous form and the hexahydrate are colourless crystals, but samples are often contaminated with iron III chloride , giving them a yellow colour. Aluminum chloride is a well-known catalyst for organic reactions. Monoclinic , mS Seed; Vaishali Sonpatki; Mark R. It reacts violently when it comes in contact with water. Its chemical formula is written as AlCl 3. View Result. This way, it has only the most useful material on its platform for the students to read from. Contents move to sidebar hide. Space group. It is used extensively in Friedel-Crafts reaction including both acylations and alkylations. It is used in pesticides and pharmaceuticals. The anhydrous without water form may be made by reacting aluminium and chlorine.
Explore the properties, production, uses, and safety concerns of Aluminum Chloride AlCl3 across industries and medicine. Aluminum Chloride, also referred to as AlCl 3 , is a crucial compound in the realm of inorganic chemistry. The compound appears as a white or pale yellow solid, depending on its purity, and has notable uses in various industrial and chemical processes.
It creates a hissing sound when it comes in contact with water. Lattice constant. Hydrogen bonds link the cation and anions. Formula units Z. Gmelin Reference. Die Struktur von Chromchlorid- und Aluminiumchloridhexahydrat". DyCl 2 DyCl 3. In some studies, aluminum chloride has been found to be a neurotoxin that can be destructive to nerve tissues and can cause permanent damage. PaCl 4 PaCl 5. Aluminium chloride monomer belongs to the point group D 3h in its monomeric form and D 2h in its dimeric form. Aluminium fluoride Aluminium bromide Aluminium iodide.

Between us speaking, I would go another by.