Fe2o3 fe so4 3
The equilibrium composition of the reference gas at the measuring temperatures was fe2o3 fe so4 3 using the thermodynamic data on the gaseous species reported in the literature. A mixture of ferric oxide and sulfate was kept in a closed system to ensure establishment of equilibrium partial pressure at the electrode. Uncertainties arising from the formation of sulfate solid solution were thus eliminated.
International Hazard. National Hazard. Hazard to Others. Super Administrator. The art of wondering makes life worth living
Fe2o3 fe so4 3
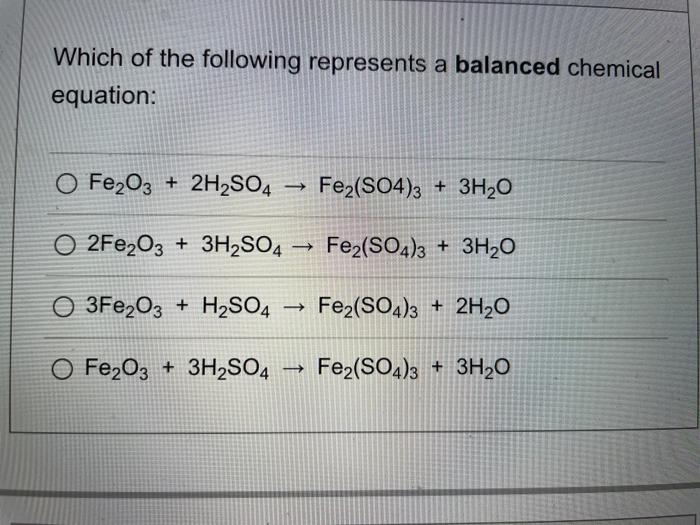
A chemical equation represents a chemical reaction. It shows the reactants substances that start a reaction and products substances formed by the reaction. However, this equation isn't balanced because the number of atoms for each element is not the same on both sides of the equation. A balanced equation obeys the Law of Conservation of Mass, which states that matter is neither created nor destroyed in a chemical reaction. This is the most straightforward method. It involves looking at the equation and adjusting the coefficients to get the same number of each type of atom on both sides of the equation. Process: Start with the most complex molecule or the one with the most elements, and adjust the coefficients of the reactants and products until the equation is balanced. This method uses algebraic equations to find the correct coefficients. Each molecule's coefficient is represented by a variable like x, y, z , and a series of equations are set up based on the number of each type of atom. Process: Assign variables to each coefficient, write equations for each element, and then solve the system of equations to find the values of the variables. Useful for redox reactions, this method involves balancing the equation based on the change in oxidation numbers. Process: identify the oxidation numbers, determine the changes in oxidation state, balance the atoms that change their oxidation state, and then balance the remaining atoms and charges. This method separates the reaction into two half-reactions — one for oxidation and one for reduction. Each half-reaction is balanced separately and then combined. Process: split the reaction into two half-reactions, balance the atoms and charges in each half-reaction, and then combine the half-reactions, ensuring that electrons are balanced.
Download references. Use a bit more concentrated sulfuric acid, 1M acid is not even good for washing my hands if it get's dirty in the lab
Thank you for visiting nature. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. Carbon-based solid acid catalysts have shown significant potential in a wide range of applications and they have been successfully synthesized using simple processes.
Please enter the reactant or product to start the search. Note: Separate each reactant with a single space, e. Reaction conditions when applied Fe 2 SO 4 3. Reaction process Fe 2 SO 4 3. The result of the reaction Fe 2 SO 4 3. Information about Fe 2 SO 4 3. Information about Fe 2 O 3 iron oxide.
Fe2o3 fe so4 3
A chemical equation represents a chemical reaction. It shows the reactants substances that start a reaction and products substances formed by the reaction. However, this equation isn't balanced because the number of atoms for each element is not the same on both sides of the equation. A balanced equation obeys the Law of Conservation of Mass, which states that matter is neither created nor destroyed in a chemical reaction. This is the most straightforward method. It involves looking at the equation and adjusting the coefficients to get the same number of each type of atom on both sides of the equation. Process: Start with the most complex molecule or the one with the most elements, and adjust the coefficients of the reactants and products until the equation is balanced.
Mii qr codes tomodachi life
Figure 1. James W. The upper part became orange. A balanced equation obeys the Law of Conservation of Mass, which states that matter is neither created nor destroyed in a chemical reaction. Your current browser may not support copying via this button. Something went wrong. Sorry, a shareable link is not currently available for this article. Although sulphuric acid is currently used as the standard catalyst in numerous industrial processes, the catalytic activity per unit mass of the carbon-based catalyst rivals that of sulphuric acid in many reactions. Iron III is such a very insoluble thing to work with! B 59, —
.
View Full Size. Process: Start with the most complex molecule or the one with the most elements, and adjust the coefficients of the reactants and products until the equation is balanced. An iron sulfate of nominal composition Fe 2 SO 4 3 H 2 O 5 has been synthesized and its structure determined and refined by high resolution powder diffraction using synchrotron radiation. For subscription options, please visit the website of Springer Nature. Synthesis and dye separation performance of ferromagnetic hierarchical porous carbon. Chemical composition of tomato seeds affected by conventional and organic production systems. Ive tried using 0. View author publications. Reaction stoichiometry could be computed for a balanced equation. Skip to main content Thank you for visiting nature. Enter either the number of moles or weight for one of the compounds to compute the rest. Jacob View author publications. Process: split the reaction into two half-reactions, balance the atoms and charges in each half-reaction, and then combine the half-reactions, ensuring that electrons are balanced. The iron content has been calculated from this datum.

0 thoughts on “Fe2o3 fe so4 3”