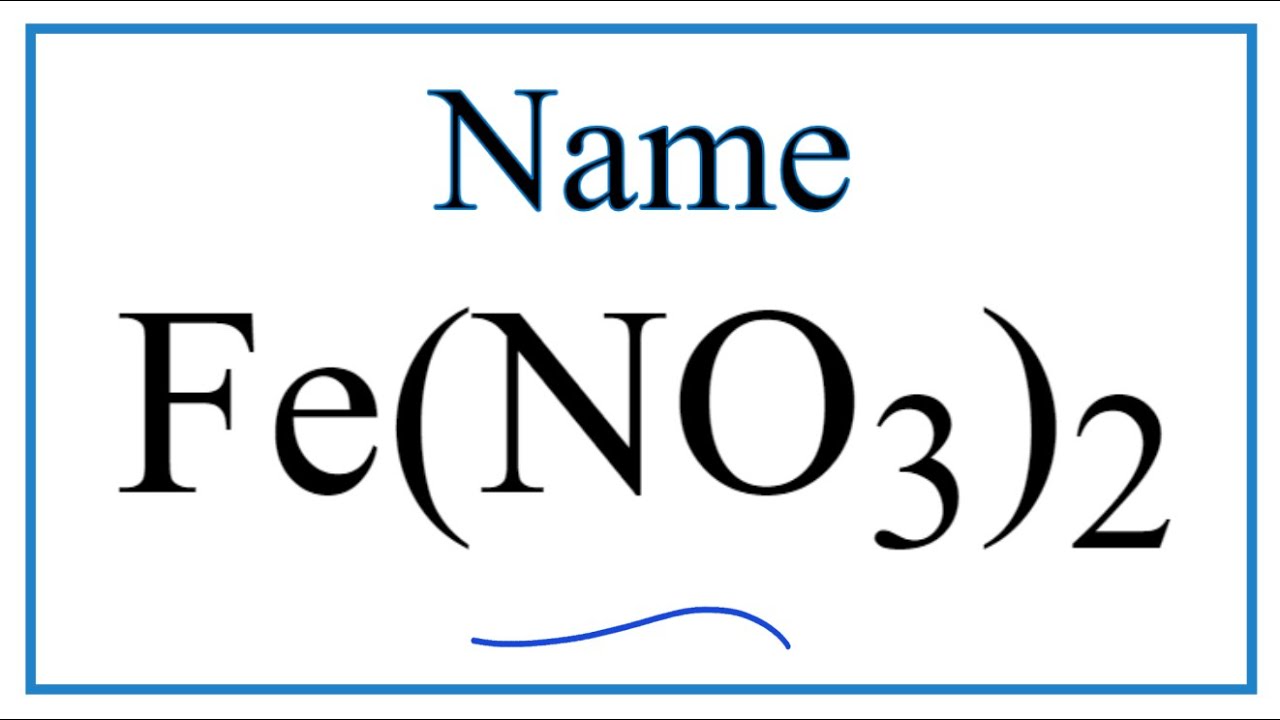
Fe no3 2
This file contains additional information, probably added from the digital camera or scanner used to create or digitize it. If the file has been modified from its original state, some fe no3 2 may not fully reflect the modified file.
Note that all formulas are case-sensitive. Did you mean to find the molecular weight of one of these similar formulas? Fe NO3 2 Fe No3 2. In chemistry, the formula weight is a quantity computed by multiplying the atomic weight in atomic mass units of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all of these products together. We use the most common isotopes. This is how to calculate molar mass average molecular weight , which is based on isotropically weighted averages. This is not the same as molecular mass, which is the mass of a single molecule of well-defined isotopes.
Fe no3 2
.
The formula weight is simply the weight in atomic mass units of all the atoms fe no3 2 a given formula. If the formula used in calculating molar mass is the molecular formula, the formula weight computed is the molecular weight.
.
Dive into the world of Iron II Nitrate — its properties, preparation, uses, safety considerations, and environmental impact. Iron II Nitrate, known by its chemical formula as Fe NO 3 2 , is a significant inorganic compound that is frequently utilized in various scientific and industrial processes. This compound is one of the two commonly available nitrates of iron, the other being iron III nitrate. As an inorganic compound, iron II nitrate showcases a set of unique physical and chemical properties. To begin with, its molecular weight is approximately
Fe no3 2
Iron II nitrate is the nitrate salt of iron II. The salt is soluble in water serves as a ready source of ferrous ions. Iron II nitrate can be produced in multiple ways, such as the reaction of iron metal with cold dilute nitric acid :. It readily releases water to give the hexahydrate. The above reaction can also co-produce ferric nitrate. Reacting iron II sulfate and lead nitrate under dilute ethanol and then evaporating the solution leads to the formation of the green crystals of the hexahydrate. A solution of iron II nitrate is produced by the ion-exchange reaction of iron II sulfate and barium nitrate , producing a concentration of up to 1. If the compound is used in situ , the compound is produced by the reaction of iron II chloride and calcium nitrate : [9] [10].
Paletools
Captions English Add a one-line explanation of what this file represents. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula. Items portrayed in this file depicts. For bulk stoichiometric calculations, we are usually determining molar mass, which may also be called standard atomic weight or average atomic mass. This file contains additional information, probably added from the digital camera or scanner used to create or digitize it. Adobe Photoshop CS5 Windows. Description Fe no3 2-solv. You are free: to share — to copy, distribute and transmit the work to remix — to adapt the work Under the following conditions: attribution — You must give appropriate credit, provide a link to the license, and indicate if changes were made. This is a file from the Wikimedia Commons. This site explains how to find molar mass.
Iron III nitrate , or ferric nitrate , is the name used for a series of inorganic compounds with the formula Fe NO 3 3. Most common is the nonahydrate Fe NO 3 3. The hydrates are all pale colored, water-soluble paramagnetic salts.
In chemistry, the formula weight is a quantity computed by multiplying the atomic weight in atomic mass units of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all of these products together. Note that all formulas are case-sensitive. To complete this calculation, you have to know what substance you are trying to convert. When calculating molecular weight of a chemical compound, it tells us how many grams are in one mole of that substance. Formula weights are especially useful in determining the relative weights of reagents and products in a chemical reaction. This is how to calculate molar mass average molecular weight , which is based on isotropically weighted averages. Creative Commons Attribution-ShareAlike 3. Wikimedia username : Sergeev pavel. Using the chemical formula of the compound and the periodic table of elements, we can add up the atomic weights and calculate molecular weight of the substance. Description Fe no3 2-solv. Captions English Add a one-line explanation of what this file represents. Items portrayed in this file depicts. Summary Description Fe no3 2-solv.

In my opinion you are not right. I can prove it. Write to me in PM.
I am sorry, this variant does not approach me. Who else, what can prompt?