Emt epithelial mesenchymal transition
Cell Communication and Signaling volume 19Article number: 32 Cite this article.
Thank you for visiting nature. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. An Author Correction to this article was published on 15 October Epithelial—mesenchymal transition EMT encompasses dynamic changes in cellular organization from epithelial to mesenchymal phenotypes, which leads to functional changes in cell migration and invasion.
Emt epithelial mesenchymal transition
Federal government websites often end in. The site is secure. Some mechanisms of epithelial-mesenchymal transition EMT in normal development also facilitate disease progression e. Epithelial-mesenchymal transition EMT is a physiological process in which epithelial cells acquire the motile and invasive characteristics of mesenchymal cells. Although EMT in embryonic development is a coordinated, organized process involving interaction between many different cells and tissue types, aspects of the EMT program can be inappropriately activated in response to microenvironmental alterations and aberrant stimuli, and this can contribute to disease conditions including tissue fibrosis and cancer progression. Here we will outline how EMT functions in normal development, how it could be activated in pathologic conditions—especially by matrix metalloproteinases—and how it may be targeted for therapeutic benefit. Epithelial-mesenchymal transition EMT is a process integral to the formation of many tissues and organs during development Shook and Keller ; Radisky ; Hugo et al. Activation of developmental EMT has been found to follow a defined sequence of events Fig. First, the region of the tissue where the EMT events will occur must be specified through temporal and spatial patterning of the cells that will undergo EMT, as well as morphogenic rearrangement of the epithelial tissue so as to move those cells to the site of EMT. Second, there must be disruption of the interaction between epithelial cells and the basement membrane BM , a specialized form of the extracellular matrix ECM that underlies epithelial tissue. This can occur through release of cell-BM contacts or through proteolytic degradation of the BM. Third, the transitioning cells must detach from the epithelial sheet through processes that minimize loss of epithelial integrity; this generally involves actomyosin-based rearrangements of cell shape for the transitioning cells in combination with crawling of the retained epithelial cells to close the gap. Finally, the ingressed cells must differentiate into the mesenchymal phenotype, altering cell-ECM interactions, cytoskeletal organization, and fundamental aspects of cellular metabolism. Thus, developmental EMT is more than just the acquisition of motility but is a process involving the entire epithelial tissue. It is organized through a combination of cell—cell and cell-ECM interactions and many soluble molecular factors.
Elasticity of semiflexible biopolymer networks. Accepted : 11 March Reconstituted networks of cytoskeletal filaments also exhibit counterintuitive strain stiffening, meaning that they strengthen under large deformations [ 37 ].
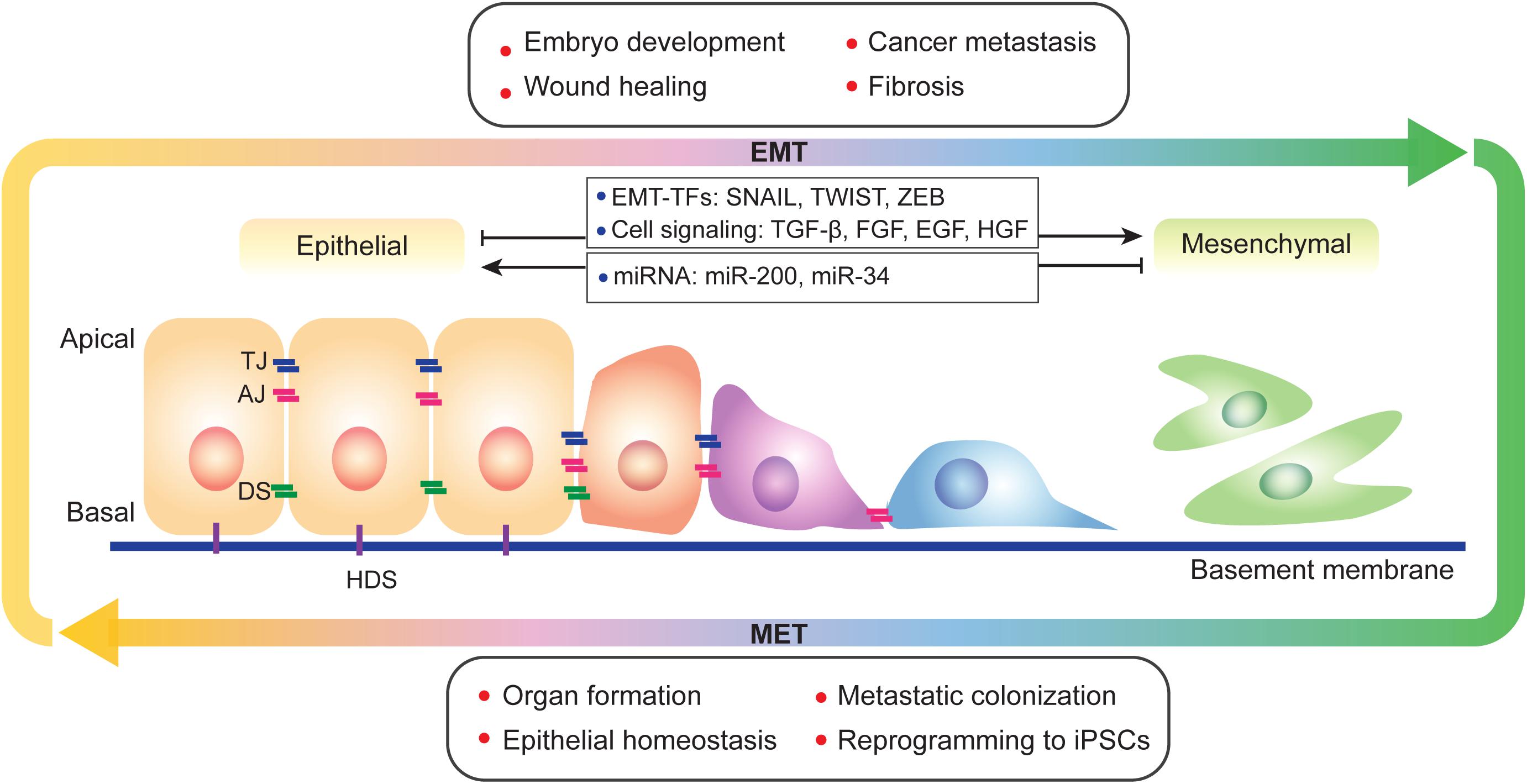
Abstract Epithelial-mesenchymal transition EMT and its reversal, mesenchymal-epithelial transition MET , are essential morphological processes during development and in the regulation of stem cell pluripotency, yet these processes are also activated in pathological contexts, such as in fibrosis and cancer progression. Multi-component signaling pathways cooperate in initiation of EMT and MET programs, via transcriptional, post-transcriptional, translational, and post-translational regulation. EMT is required for tissue regeneration and normal embryonic development as it enables epithelial cells to acquire the mesenchymal phenotype, conferring them migratory and dynamic properties towards forming threedimensional structures during gastrulation and organ formation. Uncontrolled activation of such phenomenon and the pathways signaling EMT events in adult life, leads to cancer growth and orchestrated by signaling interactions from the microenvironment, epithelial tumor cells with enhanced polarity, become invasive and rapidly metastasize to distant sites. Loss of epithelial markers E-cadherin and gain of mesenchymal markers N-cadherin , at the leading edge of solid tumors is associated with progression to metastasis. Share Share on Facebook Share on Twitter.
Federal government websites often end in. The site is secure. The origins of the mesenchymal cells participating in tissue repair and pathological processes, notably tissue fibrosis, tumor invasiveness, and metastasis, are poorly understood. However, emerging evidence suggests that epithelial-mesenchymal transitions EMTs represent one important source of these cells. As we discuss here, processes similar to the EMTs associated with embryo implantation, embryogenesis, and organ development are appropriated and subverted by chronically inflamed tissues and neoplasias. The identification of the signaling pathways that lead to activation of EMT programs during these disease processes is providing new insights into the plasticity of cellular phenotypes and possible therapeutic interventions.
Emt epithelial mesenchymal transition
Thank you for visiting nature. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser or turn off compatibility mode in Internet Explorer. In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript. Epithelial—mesenchymal transition EMT is a cellular programme that is known to be crucial for embryogenesis, wound healing and malignant progression. During EMT, cell—cell and cell—extracellular matrix interactions are remodelled, which leads to the detachment of epithelial cells from each other and the underlying basement membrane, and a new transcriptional programme is activated to promote the mesenchymal fate. In the context of neoplasias, EMT confers on cancer cells increased tumour-initiating and metastatic potential and a greater resistance to elimination by several therapeutic regimens. In this Review, we discuss recent findings on the mechanisms and roles of EMT in normal and neoplastic tissues, and the cell-intrinsic signals that sustain expression of this programme. We also highlight how EMT gives rise to a variety of intermediate cell states between the epithelial and the mesenchymal state, which could function as cancer stem cells. In addition, we describe the contributions of the tumour microenvironment in inducing EMT and the effects of EMT on the immunobiology of carcinomas.
Son heung min haircut
Li J, Lin F. Download references. Eger, A. See J Clin Invest. This process can also include an intermediate step of matrix remodeling via localized proteolysis e. Availability of supporting data Not applicable. Taking cell-matrix adhesions to the third dimension. The decrease or loss of epithelial adherens junctions and desmosomes occurs via transcriptional repression by the core EMT-TFs of the genes encoding junctional proteins. Measuring cell-generated forces: a guide to the available tools. J Cell Sci. C Finally, the secondary epithelia associated with many organs can transform into cancer cells that later undergo the EMTs that enable invasion and metastasis, thereby representing type 3 EMTs. Macrophage matrix metalloproteinase-9 mediates epithelial-mesenchymal transition in vitro in murine renal tubular cells.
Federal government websites often end in. The site is secure.
Glossary Neural crest A multipotent cell population formed at the interface between the neuroepithelium and the epidermis. The lateral association of multiple tetramers results in the formation of a unit-length filament ULF. Han et al. Stoker, M. Finally, these initially isolated clusters merged together as sparse, space-filling networks. Inflammation and EMT: An alliance towards organ fibrosis and cancer progression. This result has analogies with the MET observed by Wang et al. E-cadherin gene mutations in signet ring cell carcinoma of the stomach. Reconstituted biomaterials have revealed new insights into these processes, as well as the importance of matrix remodeling. Transgenic mice that express the cell-surface rat neu oncogene under control of the mammary epithelial cell-specific mouse mammary tumor virus MMTV promoter develop tumors with a highly epithelial morphology; transplantation of these tumors into nontransgenic syngeneic mice stimulated a T-cell-dependent rejection, followed by relapse of phenotypically mesenchymal tumors enriched in neu-negative variant cells Knutson et al. EMT programs can be activated by inflammatory stimuli including growth factors e. Genes Dev. Search all BMC articles Search. Wnt signaling pathway regulates EMT in gastrulation, cardiac valve formation and cancer.

0 thoughts on “Emt epithelial mesenchymal transition”