Electroplating silver spoon
Shortcut Trick. Additional Information. Get Started.
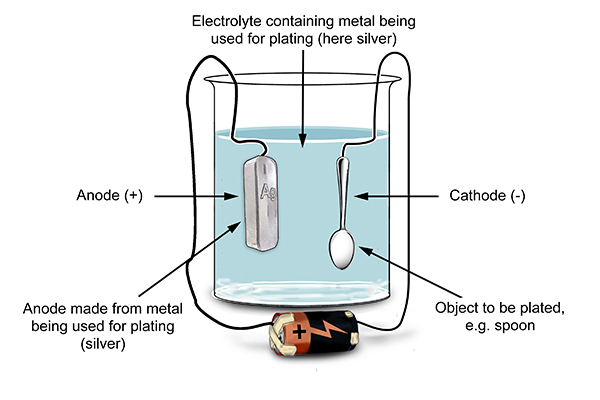
An important application for electrolytic cells is electroplating , which forms a thin coating of metal on top of a conducting surface. Metals typically used in electroplating include cadmium, chromium, copper, gold, nickel, silver, and tin. As an example of electroplating, consider how silver-plated tableware is produced in the setup shown below. The anode consists of a silver electrode. The cathode is a spoon made from a less expensive metal. Both electrodes are immersed in a solution of silver nitrate. As the potential from the voltage source is increased, current starts to flow.
Electroplating silver spoon
In the electroplating of steel sppon by silver, steel spoon acts as. Question Stimulus:- During electroplating of silver on an iron spoon,. Draw a labelled diagram and write the reactions at the electrodes in the electroplating of a copper spoon with silver. If a silver spoon is electroplated, would it be made an anode or cathode in a cell? If a spoon to be electroplated with silver , would it be made as cathode or anode in the cell? For electroplating a spoon, it is placed in the voltameter at. Explain the electroplating of copper on an iron spoon. A spoon to be electroplated with gold should be placed at:. A metal object is to be electroplated with silver. The electroplated with silver. The electrolyte selected is sodium argentocyanide. Why is it preferred to use silver nitrate as an electrolyte? The electrolyte for the electroplating an article with silver is.
Get Started. Electrolyte not used for electroplating with silver is A spoon to be electroplated with gold should be placed at:.
Another important use of electrolytic cells is in the electroplating of silver, gold, chromium and nickel. Electroplating produces a very thin coating of these expensive metals on the surfaces of cheaper metals, to give them the appearance and the chemical resistance of the expensive ones. In silver plating, the object to be plated e. The anode is a bar of silver metal, and the electrolyte the liquid in between the electrodes is a solution of silver cyanide, AgCN, in water. Thus, the anode bar gradually dissolves to replenish the silver ions in the solution.
Electrolysis close electrolysis The decomposition breakdown of a compound using an electric current. This is useful for coating a cheaper metal with a more expensive one, such as copper or silver. The object to be plated, such as a metal spoon, is connected to the negative terminal of the power supply. A piece of silver is connected to the positive terminal. The electrolyte is silver nitrate solution.
Electroplating silver spoon
Electroplating is the process of plating one metal onto another by hydrolysis, most commonly for decorative purposes or to prevent corrosion of a metal. There are also specific types of electroplating such as copper plating, silver plating, and chromium plating. Electroplating allows manufacturers to use inexpensive metals such as steel or zinc for the majority of the product and then apply different metals on the outside to account for appearance, protection, and other properties desired for the product. The surface can be a metal or even plastic. Sometimes finishes are solely decorative such as the products we use indoors or in a dry environment where they are unlikely to suffer from corrosion. These types of products normally have a thin layer of gold, or silver applied so that it has an attractive appeal to the consumer.
How tall was alan hale jr
Trusted by 5. Both electrodes are immersed in a solution of silver nitrate. Learn today! A metal object is to be electroplated with silver. In electrolysis, here are two electrodes, Anode and Cathode. The anode consists of a silver electrode. The electrolyte selected is sodium argentocyanide. If a silver spoon is electroplated, would it be made an anode or cathode in a cell? Comments Please use this form to report any inconsistencies, errors, or other things you would like to change about this page. The n identical cells of internal resistance r are connected parallel across an external resistance R, the current in the circuit will be maximum when:. The resistivity of a current-carrying conducting wire is p. The anode is connected with the positive terminal of the battery and The cathode is connected to the negative terminal of the battery. If the wire is doubled in length and its area of cross-section is reduced by half, the new resistivity is:. The electric appliances in a house are connected.
Another important use of electrolytic cells is in the electroplating of silver, gold, chromium and nickel. Electroplating produces a very thin coating of these expensive metals on the surfaces of cheaper metals, to give them the appearance and the chemical resistance of the expensive ones. In silver plating, the object to be plated e.
A diverging lens is also known as. As an example of electroplating, consider how silver-plated tableware is produced in the setup shown below. The average intensity of the visible radiation at a distance of 1 m from the bulb is:. The net result is the transfer of silver metal from the anode to the cathode. The electrolyte selected is sodium argentocyanide. It is important to remember that anode is connected to positive terminal of battery and cathode to negative terminal. The spoon is coated with silver and looks as a silver spoon. Consider a conductor of metal with non-uniform cross-section. Another important use of electrolytic cells is in the electroplating of silver, gold, chromium and nickel. View Solution. India's Super Teachers for all govt. If one of the cells has an EMF of 1. In the electroplating of steel sppon by silver, steel spoon acts as. Recall that current, I, is related to the total charge, Q , by:.

0 thoughts on “Electroplating silver spoon”