Electron withdrawing groups list
Hey there! We receieved your request.
Open navigation menu. Close suggestions Search Search. User Settings. Skip carousel. Carousel Previous. Carousel Next.
Electron withdrawing groups list
Homework problems? Exam preparation? Trying to grasp a concept or just brushing up the basics? Our proven video lessons ease you through problems quickly, and you get tonnes of friendly practice on questions that trip students up on tests and finals. Our personalized learning platform enables you to instantly find the exact walkthrough to your specific type of question. Activate unlimited help now! You can still navigate around the site and check out our free content, but some functionality, such as sign up, will not work. If you do have javascript enabled there may have been a loading error; try refreshing your browser. Learn and Practice With Ease Our proven video lessons ease you through problems quickly, and you get tonnes of friendly practice on questions that trip students up on tests and finals. Instant and Unlimited Help Our personalized learning platform enables you to instantly find the exact walkthrough to your specific type of question. Get the most by viewing this topic in your current grade.
Thank you for registering. The -M effect of the nitroso group As a result, the nitroso group is a deactivator.
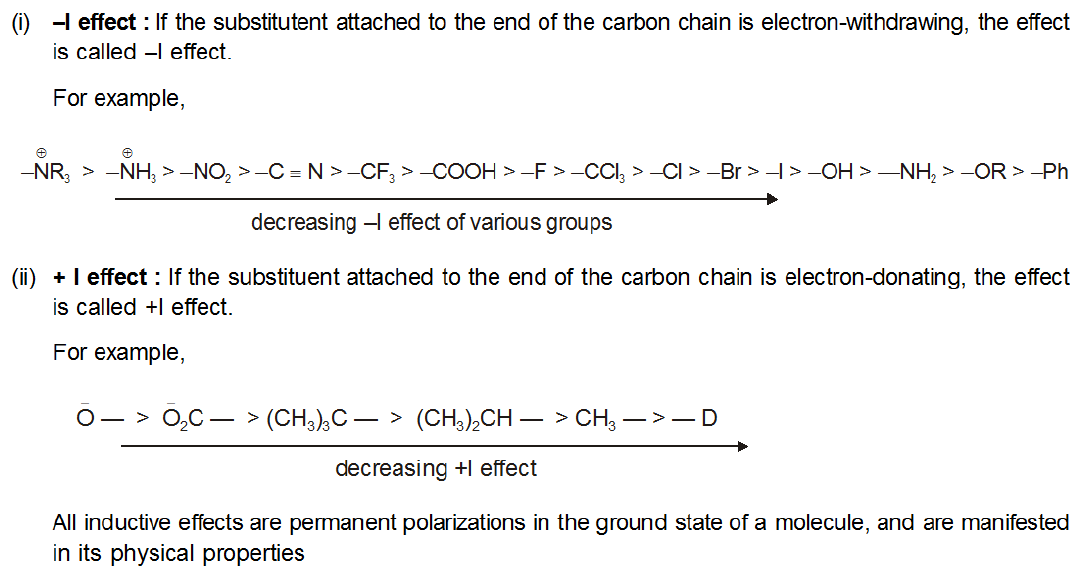
In electrophilic aromatic substitution reactions, existing substituent groups on the aromatic ring influence the overall reaction rate or have a directing effect on positional isomer of the products that are formed. EDGs are therefore often known as activating groups , though steric effects can interfere with the reaction. An electron withdrawing group EWG will have the opposite effect on the nucleophilicity of the ring. EDGs and EWGs also determine the positions relative to themselves on the aromatic ring where substitution reactions are most likely to take place. Electron donating groups are typically divided into three levels of activating ability The "extreme" category can be seen as "strong". Electron withdrawing groups are assigned to similar groupings.
The above reaction would more readily proceed if the electrophilicity of the carbonyl carbon were enhanced. This may be achieved through electron withdrawal via the R group. The ether -OMe , the methyl -Me , and the hydroxyl -OH , would all produce a electron-donating effect, and are thus incorrect answers. The nitro group -NO 2 , and the positively charged, tetra-substituted amino group consider the structure once this trimethyl amino group is connected to the aryl ring are both electron-withdrawing. As the trimethyl amino group will have an overall positive charge and the nitro group is neutral overall , the trimethyl amino group is the stronger electron-withdrawing moiety, and is thus the correct answer. If you've found an issue with this question, please let us know. With the help of the community we can continue to improve our educational resources.
Electron withdrawing groups list
In electrophilic aromatic substitution reactions, existing substituent groups on the aromatic ring influence the overall reaction rate or have a directing effect on positional isomer of the products that are formed. EDGs are therefore often known as activating groups , though steric effects can interfere with the reaction. An electron withdrawing group EWG will have the opposite effect on the nucleophilicity of the ring. EDGs and EWGs also determine the positions relative to themselves on the aromatic ring where substitution reactions are most likely to take place. Electron donating groups are typically divided into three levels of activating ability The "extreme" category can be seen as "strong".
Univision deportes
Complete Self Study Packages. EDGs are therefore often known as activating groups , though steric effects can interfere with the reaction. London: Wiley. Organic chemistry. Our personalized learning platform enables you to instantly find the exact walkthrough to your specific type of question. Exam preparation? The effect is illustrated for electrophilic aromatic substitutions with alkyl substituents of differing steric demand for electrophilic aromatic nitration. Notice that iodobenzene is still less reactive than fluorobenzene because polarizability plays a role as well. View courses by askIITians. For example, aniline has resonance structures with negative charges around the ring system: The amino group can donate electron density through resonance.
Electrophilic Aromatic Substitution: Introduction.
London: Cambridge University Press. Inductively, the negatively charged carboxylate ion moderately repels the electrons in the bond attaching it to the ring. This is normally seen as a weak effect due to: Alkyl groups -R Aromatic ring substituents -C 6 H 5 A group with a negative inductive effect -I decreases electron density on the carbon atom by polarizing the sigma bond. There is no resonance effect because there are no orbitals or electron pairs which can overlap with those of the ring. Eleazar - Quiz 3 Eleazar - Quiz 3. Get the most by viewing this topic in your current grade. With the exception of the halides, they are meta directing groups. For example, aniline has resonance structures with negative charges around the ring system:. Carousel Next. The valence orbitals of fluorine are the 2p orbitals which is the same for carbon - hence they will be very close in energy and orbital overlap will be favourable.

I think, that you commit an error. Let's discuss it. Write to me in PM.