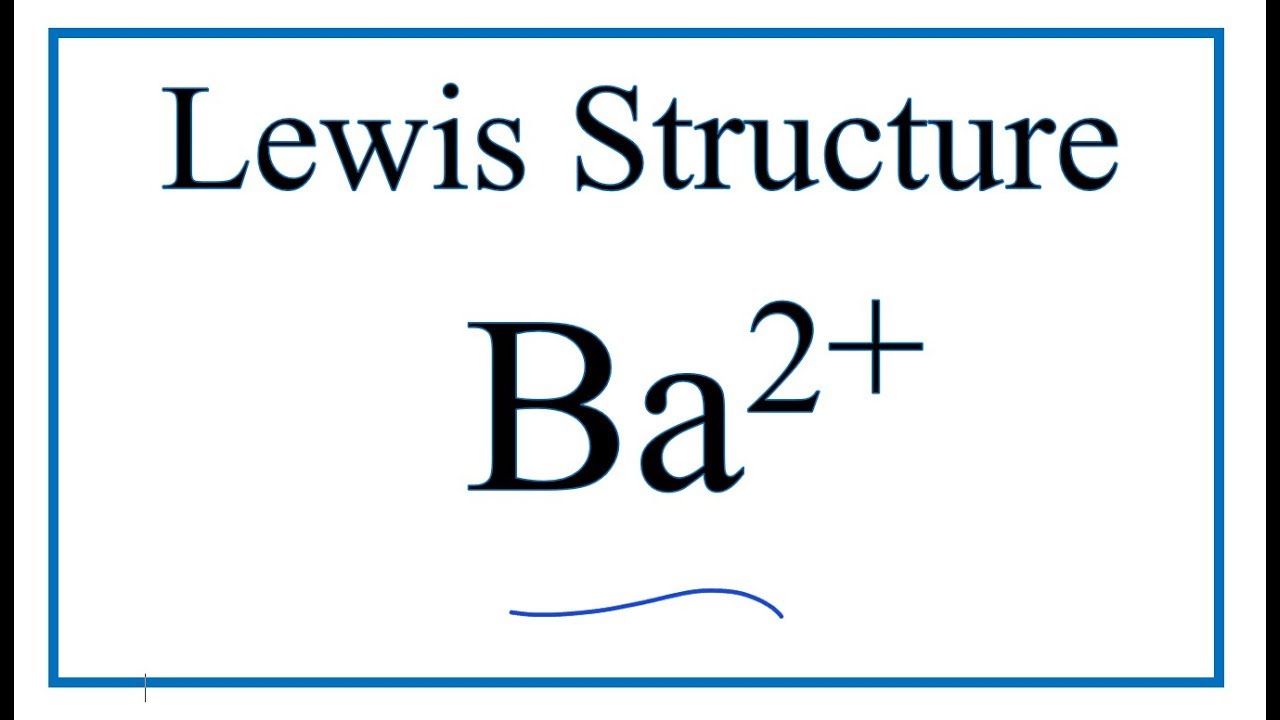
Electron dot diagram for barium
This is a file from the Wikimedia Commons. The description on its description page there is shown below. Commons is a freely licensed media file repository.
In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. A Lewis electron dot symbol or electron dot diagram or a Lewis diagram or a Lewis structure is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The number of dots equals the number of valence electrons in the atom. These dots are arranged to the right and left and above and below the symbol, with no more than two dots on a side.
Electron dot diagram for barium
Wiki User. There should be two valence electrons around the element since Strontium is in the second column of the Periodic Table and has two valence electrons filling the 5s shell. The dot structure for Rubidium is Rb with a dot on the top right of b. Rb is the short form of rubidium. The dot structure as you know can only be a max of 8 and dots are added counterclockwise. Anyway, a good counting place on the periodic table would be the second row or period. It starts with hydrogen. It and the group under it have 1 dot which is called a valence electron. As you count across, the dots increase. Beryllium has two dots.
Summary Description Lewis dot Ba. You can copy, modify, distribute and perform the work, even for commercial purposes, all without asking permission. CC licensed content, Shared previously.
To draw: the electron dot diagram for BaCl 2. A chemical bond due to the electrostatic attraction between oppositely charged ions is defined as ionic bonding. The stable configuration of any elements should contain two electrons or eight electrons in their outer shell or valence shell. The elements of barium are related to group 2. Hence, the valence electrons in these elements are 2. The elements of chlorine are related to group
Ionic bonding typically occurs when it is easy for one atom to lose one or more electrons, and for another atom to gain one or more electrons. However, some atoms will not give up or gain electrons easily. Yet they still participate in compound formation. There is another mechanism for obtaining a complete valence shell: sharing electrons. When electrons are shared between two atoms, they form a covalent bond. Let us illustrate a covalent bond by using H atoms, with the understanding that H atoms need only two electrons to fill the 1 s subshell.
Electron dot diagram for barium
Barium Lewis Dot structure would be described in this article by representing different compounds of barium. The different Lewis dot structure of different barium compounds would highlight the significant of Lewis structure in understanding their electron transfer theories. The compounds that would be drawn through Lewis structure are being listed below:. Lewis Dot structure of barium oxide shows the electron transfer process maintained in the formation of the compound. Therefore, it would be significant to draw the structure and build the idea practically. Lewis dot structure of Barium oxide is reliable in defining the nature of the bonds made by the compound, which is being represented below:. The image is denoting that the valance of Barium is two and oxygen has six valances. In the case of fulfilling octet state to get similar stability as the nearest noble gas, two valance electrons are d transferred by barium to oxygen. In the case of fulfilling octet, oxygen, the non-metal also adopts those electrons from Barium metal and makes ionic bond with barium. Read more about Ionic bonds.
Lust odyssey
Chapter 18, Problem 51R. How many electrons must be represented in the Lewis dot structure for the phosphate ion? Recommended textbooks for you. Tags Elements and Compounds Subjects. Their group has 3 dots. Privacy Policy. What is the Lewis dot structure of the nitronium ion? The dot structure as you know can only be a max of 8 and dots are added counterclockwise. Molecular Structure of Compounds. Still have questions? These barium ion and chlorine combined to form BaCl 2. Find more answers Ask your question.
A chemical element is identified by the number of protons in its nucleus, and it must collect an equal number of electrons if it is to be electrically neutral.
Author: Randall D. Briefly describe why astronauts are weightless in th What is Lewis dot and cross structure of CaCl2? The structure is then bracketed and noted with a 1- superscript. Check Your Learning What is the Lewis electron dot symbol for each element? Additional Science Textbook Solutions Find more solutions based on key concepts. Items portrayed in this file depicts. Chapter 6. The dot structure for IO4 starts with the I atom in the center. Licenses and Attributions. Lewis symbols illustrating the number of valence electrons for each element in the third period of the periodic table. It starts with hydrogen.

I apologise, but this variant does not approach me. Who else, what can prompt?