Draw the electron dot structure for ethyne
Method for calculating electron dot structure:. We can determine the electron dot structure of any given compound by the following steps:. E represents the total number of valence electrons and B.
Past Year - 3 Mark Questions. Last updated at May 29, by Teachoo. Molecular formula of Ethane is C 2 H 6. Molecular formula of Ethene is C 2 H 4. Molecular formula of Ethyne is C 2 H 2. Learn in your speed, with individual attention - Teachoo Maths 1-on-1 Class.
Draw the electron dot structure for ethyne
.
After assigning lone pairs to each atom, a double or triple bond should be drawn if necessary to satisfy the valence of the octet of each atom.
.
Menu Categories. Draw the electron dot structure of ethyne and also draw its structural formula. Tutorialspoint Simply Easy Learning. Updated on: Oct Related Articles Write the molecular formula of ethene and draw its electron dot structure. Draw the possible isomers of the compound with molecular formula C3H6O and also give their electron dot structures.
Draw the electron dot structure for ethyne
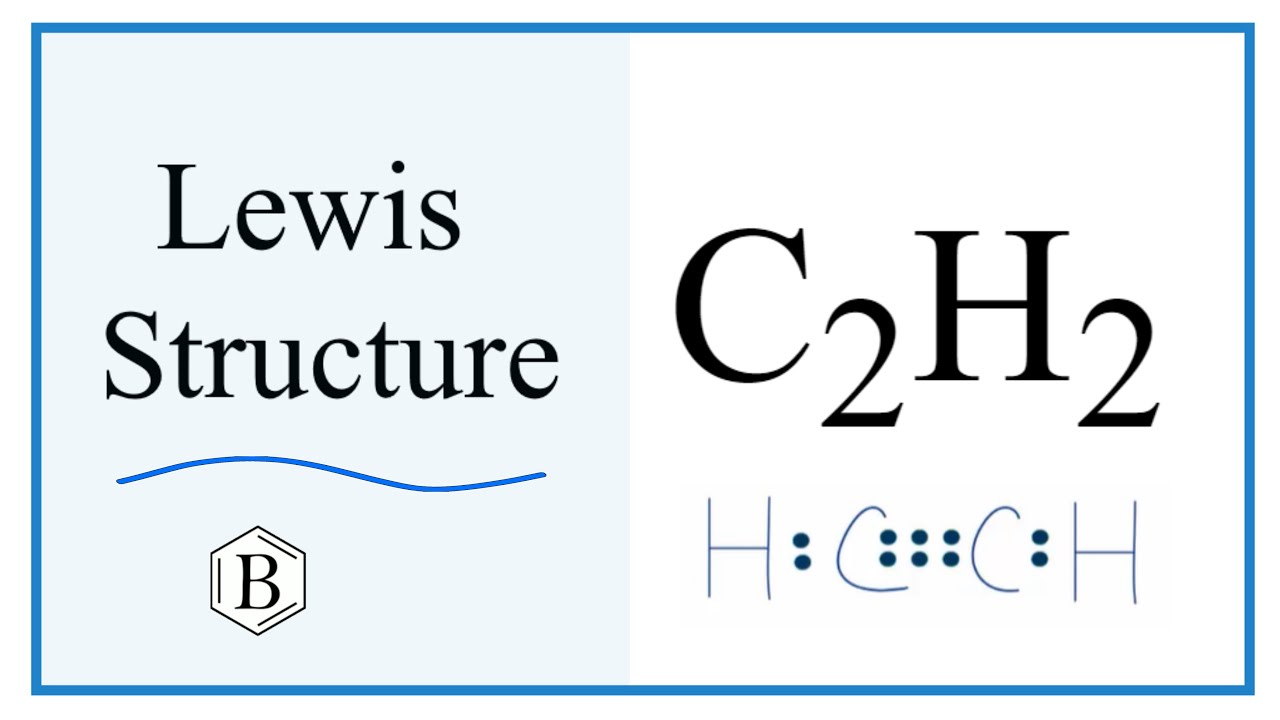
Method for calculating electron dot structure:. We can determine the electron dot structure of any given compound by the following steps:. E represents the total number of valence electrons and B. E denotes the number of electrons present in the bond pairs. The structural formula of Ethyne:. The electron dot structure of Ethyne:. Hence, the electron dot structure of ethyne C 2 H 2 has been drawn above.
Kahve sokağı menü
Method for calculating electron dot structure: We can determine the electron dot structure of any given compound by the following steps: First, the total number of valence electrons present in the molecule has to be calculated by adding the individual valencies of each atom, which can be given as: V. Write the molecular formula of the following compounds and draw their electron-dot structures: i Ethane ii Ethene iii Ethyne Answer 1. Past Year - 3 Mark Questions. We can determine the electron dot structure of any given compound by the following steps:. Write the molecular formula of ethene and draw its electron dot structure. Byju's Answer. Maths Classes. Standard X Chemistry. The structural formula of Ethyne:. Molecular formula of Ethyne is C 2 H 2. Method for calculating electron dot structure: We can determine the electron dot structure of any given compound by the following steps: First, the total number of valence electrons present in the molecule has to be calculated by adding the individual valencies of each atom, which can be given as: V. Maths Classes Teachoo Black. After assigning lone pairs to each atom, a double or triple bond should be drawn if necessary to satisfy the valence of the octet of each atom.
So far we have focused primarily on two simple types of molecular compounds: homodiatomic molecules such as H 2 , F 2 , N 2 and O 2 [22] , and binary compounds such as water. But we saw in the previous section that hydrogen and oxygen can also form another compound, namely hydrogen peroxide, H 2 O 2.
Question 30 Drawthe electron dot structure of ethyne and also draw its structural formula. Maths Classes Teachoo Black. Electron dot structure: Electron dot structures or Lewis dot formulas can only be drawn if the molecular formula of the compound is fully known. Covalent Bonding in H2, N2 and O2. E represents the total number of valence electrons and B. Standard X Chemistry. Draw the electron dot structure of ethyne and also draw its structural formula. Displaying ads are our only source of revenue. We can determine the electron dot structure of any given compound by the following steps:. Draw electron dot structure of ethyne. Now the electrons shall be assigned to the atoms in dot or cross representation to denote only the single bonding among the molecules. Write the molecular formula of the following compounds and draw their electron-dot structures: i Ethane ii Ethene iii Ethyne Answer 1. Last updated at May 29, by Teachoo.

Willingly I accept. In my opinion, it is an interesting question, I will take part in discussion. Together we can come to a right answer. I am assured.
Do not despond! More cheerfully!
I can consult you on this question and was specially registered to participate in discussion.