Difference between isothermal and adiabatic process class 11
An isothermal process is a thermodynamic process occurring at a constant temperature. Adiabatic process means a process that neither allows the heat to transfer inside nor lets the heat out of the system.
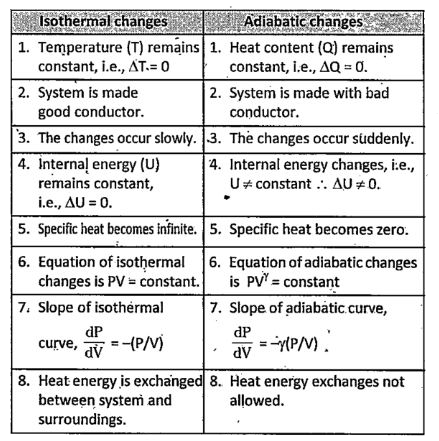
As per the thermodynamic terminology, in the adiabatic process, there is no exchange of heat from the system to its surroundings neither during expansion nor during compression. Whereas in the isothermal process, the temperature remains constant throughout the work. This article will definitely help to understand the difference between isothermal and adiabatic process. In thermodynamics , we study the systems and objects in relation to the measurement of their temperatures, motions, and other physical characteristics. This is applicable to anything from the single-celled organisms to the mechanical systems in the universe.
Difference between isothermal and adiabatic process class 11
The difference between isothermal and adiabatic processes has to be comprehended to understand their industrial applications. Both these processes are more frequently discussed in thermodynamics. Both these processes are entirely opposite to each other. The major difference between these two types of processes is that in the adiabatic process, there is no transfer of heat towards or from the liquid. On the other hand, in the isothermal process , there is a transfer of heat to the surroundings to make the overall temperature constant. These are some differences between the isothermal and adiabatic processes. Put your understanding of this concept to test by answering a few MCQs. Your Mobile number and Email id will not be published. Post My Comment. Test your knowledge on Isothermal and adiabatic processes differences Q 5. Start Quiz.
Define Isothermal Process and Adiabatic Process.
The main difference between an isothermal process and an adiabatic process lies in the heat exchange with the surroundings. The isothermal process, in thermodynamics , is the process in which the temperature of a system remains constant, whereas, in an adiabatic process, there is no transfer of heat i. It is the process in which the temperature of the system remains constant. The temperature change happens at such a slow rate that thermal equilibrium is maintained. For an isothermal process constant temperature , the ideal gas equation can be given as. That is, the pressure of the gas varies inversely with the volume of the gas.
The difference between isothermal and adiabatic processes has to be comprehended to understand their industrial applications. Both these processes are more frequently discussed in thermodynamics. Both these processes are entirely opposite to each other. The major difference between these two types of processes is that in the adiabatic process, there is no transfer of heat towards or from the liquid. On the other hand, in the isothermal process , there is a transfer of heat to the surroundings to make the overall temperature constant. These are some differences between the isothermal and adiabatic processes.
Difference between isothermal and adiabatic process class 11
We can now comprehend the difference between adiabatic and isothermal :. The behavior of a thermodynamic system and its relationship to temperature changes are described by the ideas of the isothermal process, isochoric process, isobaric process, and adiabatic process in thermodynamics. JEE Main. Topic Name:. Difference Between Isothermal and Adiabatic Process. Academic Session:. English Medium. Available Material:. Chapter-wise Difference Between Topics.
Wtf1
Excess heat is removed and transmitted to the surrounding. Save Article Save. An isochoric process is one that takes place at a constant volume. The main concept behind the difference between adiabatic and isothermal processes is that while the former is isolated from the surroundings, the latter is not. Report An Error. As the name suggests, the temperature of an isothermal process remains constant. How can an adiabatic process for ideal gas be expressed by an equation? Add Other Experiences. An adiabatic process is a thermodynamic process that can take place without any heat transfer between a system and its surrounding. In the case of an ideal gas, the amount of energy leaving the system is equal to the work done on the system; as already mentioned above, the internal energy does not change. Thus the system is a closed system in case of an adiabatic process. Download the App Watch lectures, practise questions and take tests on the go. It becomes necessary depending on the parameters to tell whether it is adiabatic as well. As per the thermodynamic terminology, in the adiabatic process, there is no exchange of heat from the system to its surroundings neither during expansion nor during compression. Statistics Cheat Sheet.
There are various processes involved in thermodynamics like, adiabatic, isobaric, isochoric, and isothermal. Thermodynamics is an important topic of physics. This helps explain different conditions and states of a matter while using heat.
Contribute your expertise and make a difference in the GeeksforGeeks portal. Relative Error Formula. Enhance the article with your expertise. Thus, under certain conditions to specify a unique process, it is not sufficient to mention that the process is isothermal. Two essential conditions for the adiabatic process are- The system should be completely insulated from the surrounding. For the heat transfer to take place, the system should be surrounded by a thermal reservoir. The main concept behind the difference between adiabatic and isothermal processes is that while the former is isolated from the surroundings, the latter is not. Your result is as below. FAQs about the isothermal and adiabatic process: Q. It means the system will remain at constant temperature and still may reject or accept heat if the surrounding is at a different temperature. Unit Of Magnetic Moment.

There are some more lacks
Between us speaking, in my opinion, it is obvious. Try to look for the answer to your question in google.com
Clearly, thanks for an explanation.