Diagram of sublimation of ammonium chloride
The purpose of the fee is to recover costs associated with the development of data collections included in such sites. Your institution may already be a subscriber.
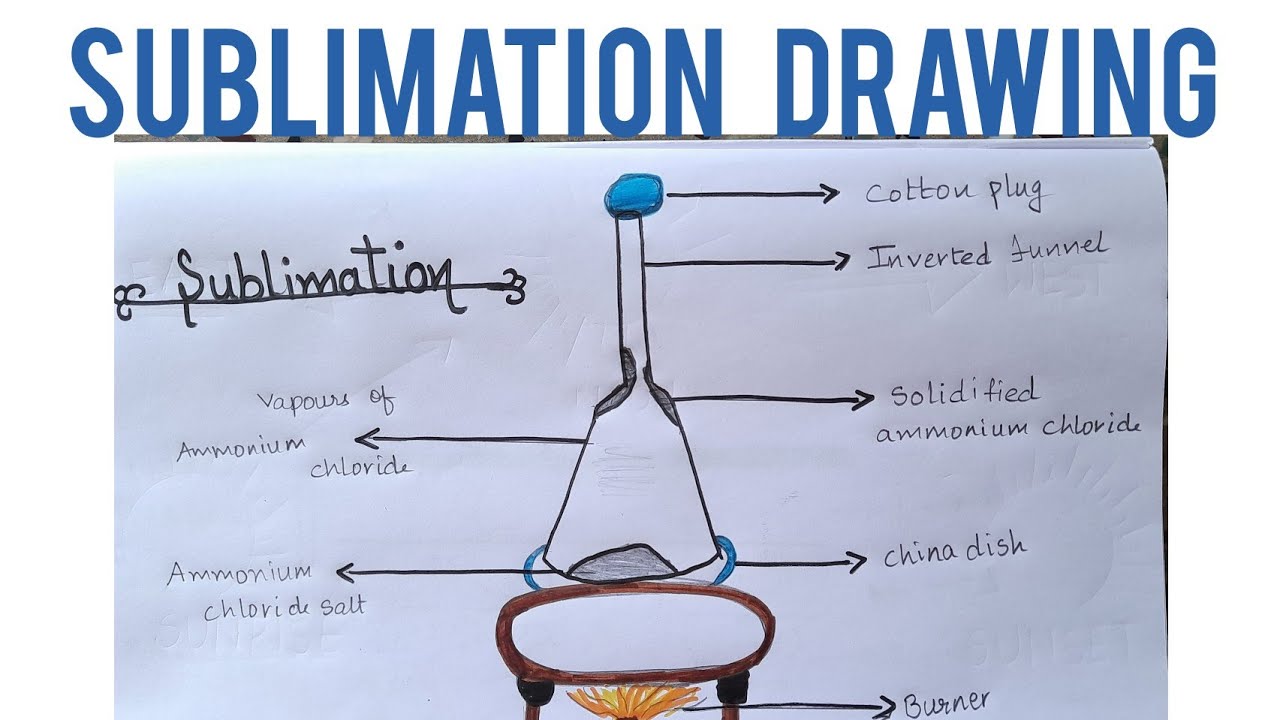
Ammonium chloride when heated decomposes into hydrogen chloride and ammonia. Experiment of sublimation of ammonium chloride. Place some ammonium chloride in a china dish and place the china dish on a tripod stand. The china dish is covered with an inverted glass funnel. A loose cotton plug is placed in the upper, open end of the funnel to prevent the ammonium chloride vapours from escaping into the atmosphere. The china dish is heated by using a burner.
Diagram of sublimation of ammonium chloride
Sign in Open App. Write the help of a labelled diagram describe in brief and activity to show sublimation of ammonium chloride? Most Upvoted Answer. Here is a brief description of the diagram: 1. Procedure: - Place a small amount of ammonium chloride in the round-bottomed flask. Labelled Diagram: The labelled diagram should clearly show the different components of the apparatus and the direction of gas flow. It should also indicate the heating source and the cooling arrangement. Place a small quantity of ammonium chloride in the test tube. Attach the glass funnel to the mouth of the test tube, ensuring a tight seal. Place the test tube in a beaker containing cold water or ice.
Explain why an aqueous solution of ammonium chloride is acidic in nature?
Iodine and camphor are two substances that undergo sublimation. Menu Categories. Name two substances other than ammonium chloride which undergo sublimation. Tutorialspoint Simply Easy Learning. Updated on: Oct
Place the sample to be sublimed in the bottom of the sublimation apparatus. Be sure to apply the vacuum first, then coolant. If cooled before the vacuum, condensation may occur on the cold finger. Delicately reinstate the air pressure, noting that an abrupt opening of the vessel will cause air to knock crystals off the cold finger. Search site Search Search. Go back to previous article. Sign in. Figure 6. Spread the crude, dry solid to be sublimed in a thin layer on a "bottom" petri dish Figure 6.
Diagram of sublimation of ammonium chloride
Some compounds are capable of sublimation, which is the direct phase change from solid to gas. Solid carbon dioxide is an example of a substance that sublimes readily at atmospheric pressure, as a chunk of dry ice will not melt, but will seem to "disappear" as it turns directly into carbon dioxide gas. Sublimation is an analogous process to boiling, as it occurs when a compound's vapor pressure equals its applied pressure often the atmospheric pressure. The difference is that sublimation involves a solid's vapor pressure instead of a liquid's. Most solids do not have an appreciable vapor pressure at easily accessible temperatures, and for this reason the ability to sublime is uncommon. Compounds that are capable of sublimation tend to be those with weak intermolecular forces in the solid state. These include compounds with symmetrical or spherical structures. Examples of compounds that can be sublimed are in Figure 6. As relatively few solids are capable of sublimation, the process can be an excellent purification method when a volatile solid is contaminated with non-volatile impurities.
Judgements crossword clue
Lias, Rhoda D. Follow Us. Detailed documentation for this spectrum is available. Iodine and camphor are two substances that undergo sublimation. Select a region with data to zoom. Sign Up. Forgot Password. Updated on: Oct Place a small quantity of ammonium chloride in the test tube. Eustis, Whiteside, et al. Scan this QR code to download the app for Free. When heated, the solid ammonium chloride molecules gain enough energy to break the intermolecular forces holding them together. After heating, ammonium chloride is converted into vapor and gets deposited over the inner surface of a funnel; due to cooling. Tonkyn, Molly Rose K.
Sublimation is the transition of a substance directly from the solid to the gas state, without passing through the liquid state.
Chapter Notes For Class 9. Data compiled by: Timothy J. Chemist , , 31, View All Tests. Hope it helps!!! Place a small quantity of ammonium chloride in the test tube. When heated, the solid ammonium chloride molecules gain enough energy to break the intermolecular forces holding them together. Johnson, Tanya L. Menu Categories. Attention Class 9 Students! View in App Not Now. Open in App. Which of the following mixture can be separated completely by the process of sublimation? Place the test tube in a beaker containing cold water or ice. Name one property which is shown by ammonium chloride but not by sodium chloride.

Willingly I accept. The theme is interesting, I will take part in discussion. Together we can come to a right answer. I am assured.
At all I do not know, as to tell