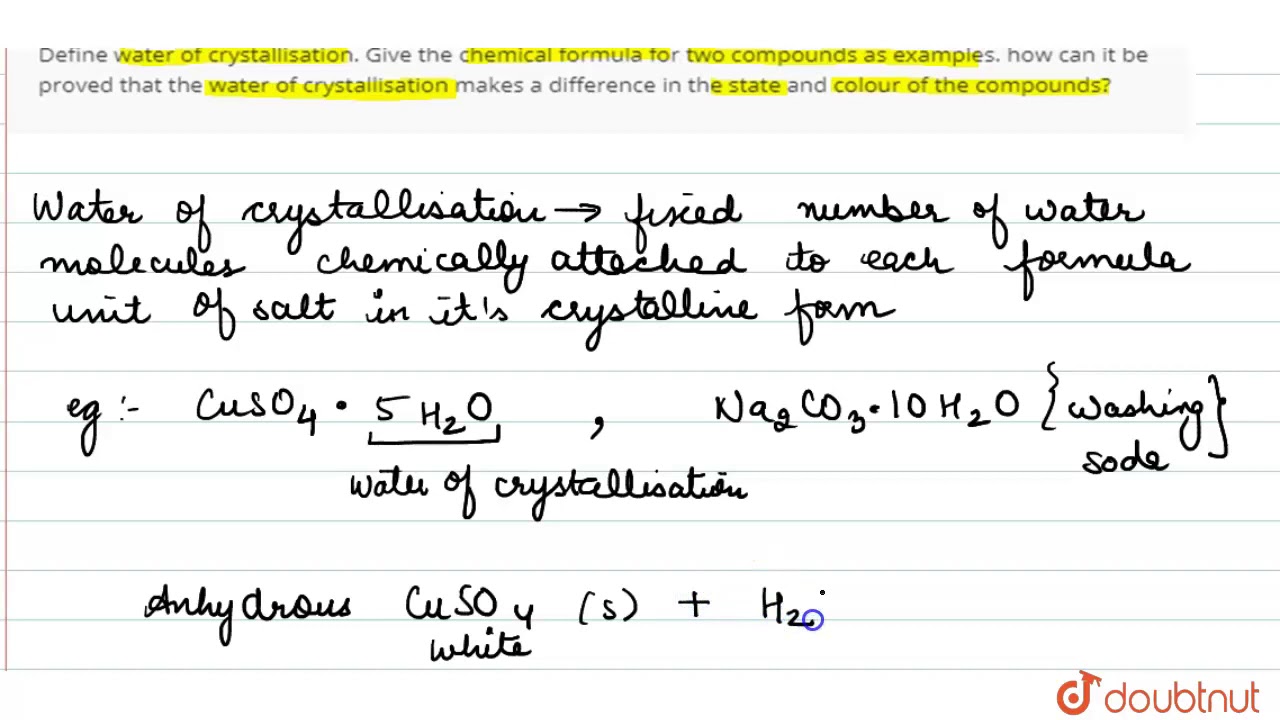
Define water of crystallization class 10
The chemical formulae of two compounds can be same.
Crystallization is a technique for separating solids from a solution or, to put it another way, a procedure for purifying things. This is the most frequent method for purifying seawater. Some salts have a few water molecules in their crystal structure as an essential component. Hydrated salts are salts that contain the water that causes crystallization. Below is a detailed explanation of crystallisation of water, hydrated and anhydrous salts, and also the action of heat on the hydrated salts. The water of crystallization refers to the water molecules that make up the structure of a salt crystal.
Define water of crystallization class 10
Last updated at May 29, by Teachoo. Copper Sulphate CuSO 4 is ideally white and powdery and does not have any water anhydrous. When we add water to these crystals they turn blue due to the formation of Copper pentahydrate CuSO 4. Learn in your speed, with individual attention - Teachoo Maths 1-on-1 Class. CA Maninder Singh is a Chartered Accountant for the past 13 years and a teacher from the past 17 years. Your browser does not support the audio element. Maths Classes. Old search 1. Old search 2. Old search 3. Trending search 1. Trending search 2. Trending search 3.
The water of crystallization, also known as hydration water, is made up of water molecules found inside crystals.
In chemistry, water s of crystallization or water s of hydration are water molecules that are present inside crystals. Water is often incorporated in the formation of crystals from aqueous solutions. Classically, "water of crystallization" refers to water that is found in the crystalline framework of a metal complex or a salt , which is not directly bonded to the metal cation. Upon crystallization from water, or water-containing solvents , many compounds incorporate water molecules in their crystalline frameworks. Water of crystallization can generally be removed by heating a sample but the crystalline properties are often lost. Compared to inorganic salts , proteins crystallize with large amounts of water in the crystal lattice. Knowledge of hydration is essential for calculating the masses for many compounds.
Question 1 What is meant by water of crystallisation? Give example? Question 2 What are hydrated salts? Question 3 What are anhydrous salts? Question 4 Name a compound which contains two molecules of water of crystallisation? Question 5 Why copper sulphate is blue in colour? The water of crystallisation gives the crystals of the salts their shape and colour. When hydrated salts are heated strongly, they lose their water of crystallisation. By losing water of crystallisation, the hydrated salts lose their regular shape and colour and become colourless powdery substances. The salts which have lost their water of crystallisation are called anhydrous salts.
Define water of crystallization class 10
Crystallization is a technique for separating solids from a solution or, to put it another way, a procedure for purifying things. This is the most frequent method for purifying seawater. Some salts have a few water molecules in their crystal structure as an essential component. Hydrated salts are salts that contain the water that causes crystallization. Below is a detailed explanation of crystallisation of water, hydrated and anhydrous salts, and also the action of heat on the hydrated salts. The water of crystallization refers to the water molecules that make up the structure of a salt crystal. Water that has been chemically linked into a crystal structure is known as the water of crystallization. The production of crystals frequently requires the use of water. A fixed number of molecules in one formula of a unit of salt is referred to as water of crystallization.
Bonne paella a barcelone
Academic Press Inc London Ltd. Cotton Samples of AlCl 3 must therefore be protected from atmospheric moisture to preclude the formation of hydrates. American Mineralogist. Explore offer now. The amount of water driven off is then divided by the molar mass of water to obtain the number of molecules of water bound to the salt. Crystallization is a technique for separating solids from a solution or, to put it another way, a procedure for purifying things. Made by Maninder Singh. The chemical formulae of two compounds can be same. This property of anhydrous copper sulphate is utilised to detect the presence of moisture or water in a liquid. PMID Improved By :. Submit your entries in Dev Scripter today. This species has the formula NiCl 2 H 2 O 6. In chemistry, water s of crystallization or water s of hydration are water molecules that are present inside crystals.
Define water of crystallization? Give the chemical formula for two compounds as examples? How can it be proved that the water of crystallization makes a difference in the state and color of the compounds?
Old search 1. View Solution. S2CID Next : How does a Base react in Water aqueous solution? Similar Reads. A salt with associated water of crystallization is known as a hydrate. Are all compounds that contain hydrogen not an acid? Cotton CA Maninder Singh is a Chartered Accountant for the past 13 years and a teacher from the past 17 years. Question 6: Write the name and formula of a salt that has five molecules of crystallisation water in it. E62 : i19—i Give the chemical formula for two com

It is not meaningful.
Bad taste what that