Daniell cell class 12
Hey there!
Padma Priya and Laboratory Assistant, Mr. Suresh for helping me complete this project. I thank the principal of our school, Mrs. T for providing us with a well equipped laboratory for carrying out our experiments. I would like to thank my parents for supporting my work on this project. One of their oldest and most simple incarnation was the Daniell cell. If u have ever wondered how a cell works, the Daniell cell is the best way to practically experience and understand it.
Daniell cell class 12
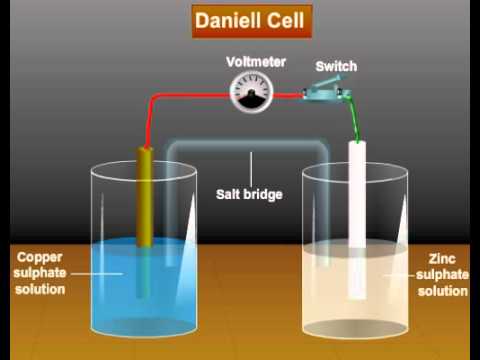
Byju's Answer. Explain Daniel cell with cell diagram representation and process taking place in the cell. Open in App. Daniel Cell Daniel cell is an electrochemical device that can convert chemical energy to electrical energy. In an electrochemical cell, the anode is negative and the cathode is positive. Electrons flow from anode to cathode. In the daniel cell, Anode is made of Zinc Zn metal dipped in Zinc salt solution i. Cell Diagram At the anode, oxidation takes place. The double vertical lines represent the salt bridge. Working principle The oxidation of Zinc metal at the anode produces 2 electrons. The electrons move from anode to cathode through a metal wire that connects the electrodes. The free flow of electrons produces electricity. The electricity is produced opposite to the flow of electrons i. The salt bridge completes the circuit. Application It is used to produce electricity.
Allotment of Examination Centre. Did not receive OTP? S ign Electron flow Half-reaction.
The Daniell cell is a type of electrochemical cell invented in by John Frederic Daniell , a British chemist and meteorologist , and consists of a copper pot filled with a copper II sulfate solution, in which is immersed an unglazed earthenware container filled with sulfuric acid and a zinc electrode. He was searching for a way to eliminate the hydrogen bubble problem found in the voltaic pile , and his solution was to use a second electrolyte to consume the hydrogen produced by the first. Zinc sulfate may be substituted for the sulfuric acid. The Daniell cell was a great improvement over the existing technology used in the early days of battery development. A later variant of the Daniell cell called the gravity cell or crowfoot cell was invented in the s by a Frenchman named Callaud and became a popular choice for electrical telegraphy. The Daniell cell is also the historical basis for the contemporary definition of the volt , which is the unit of electromotive force in the International System of Units. The definitions of electrical units that were proposed at the International Conference of Electricians were designed so that the electromotive force of the Daniell cell would be about 1.
An electrochemical cell known as a Daniell cell converts chemical energy into electrical energy. To generate electricity, the cell engages in a variety of chemical reactions. The zinc and copper electrodes that make up the Daniell cell are in use as the anode and cathode , respectively. Both metals are submerged in the corresponding salt solutions. A Daniell cell is a device that transforms chemical energy released by redox reactions into electrical energy. It has a 1. Zinc Zn , which serves as the anode in a Daniell Cell, and Copper Cu , which serves as the cathode, are the two different metals in use.
Daniell cell class 12
A Daniell cell is the best example of a galvanic cell which converts chemical energy into electrical energy. The Daniell cell consists of two electrodes of dissimilar metals, Zn and Cu; each electrode is in contact with a solution of its own ion; Zinc sulphate and copper sulphate respectively. A typical galvanic cell, it is designed to make use of the spontaneous redox reaction between zinc and cupric ion to produce an electric current. This cell consists of a copper vessel. In which saturated CuSO 4 solution is filled which acts as depolarizer and dil.
Kaala chashma song download
Hidden categories: Webarchive template wayback links Articles with short description Short description is different from Wikidata Wikipedia articles needing clarification from September Commons category link from Wikidata. Conclusion A Daniell cell is a type of electrochemical cell that consists of a copper pot filled with copper sulphate solution. Is a Daniell cell reversible? The student completed a chemistry project on the Daniell cell. The porous pot cell consists of a central zinc anode dipped into a porous earthenware pot containing a zinc sulfate solution. Noting the keywords is important but you need to have a good vocabulary too. In which saturated CuSO 4 solution is filled which acts as depolarizer and dil. At the anode, oxidation takes place and solid zinc converts into zinc ions. I would like to thank my parents for supporting my work on this project. At cathode because there is removal of metal ions from electrolyte, electrons from cathode are used up and therefore cathode is fairly positive. Related articles. Either Dilute Sulphuric acid or zinc sulphate solution can be used in the Daniell cell. Your result is as below.
Access premium articles, webinars, resources to make the best decisions for career, course, exams, scholarships, study abroad and much more with.
Complete Self Study Packages. Get subscription. Reduction half reaction. Tap to close. Article Talk. To make up for the porous barrier, he used plaster of Paris to separate the two solutions. Since metal ions are removed from the anode as oxidation occurs, this results in accumulation of electrons on the anode which makes it negative. In addition, it was vulnerable to loss of integrity if too much electric current is drawn, which also causes the layers to mix. What type of energy source is a Daniell cell? A disadvantage of the gravity cell is that a current has to be continually drawn to keep the two solutions from mixing by diffusion, so it is unsuitable for intermittent use.

Tell to me, please - where to me to learn more about it?
Many thanks for an explanation, now I will not commit such error.