Cr2 so4 3
With finding oxidation numbersyou have to know the common oxidation states of an element. There might be some little algebra involved.
Additionally, ill-defined but commercially important "basic chromium sulfates" are known. These salts are usually either violet or green solids that are soluble in water. It is commonly used in tanning leather. A variety of other chromium III sulfates are known, but also contain hydroxide or oxide ligands. Other chromium III hydroxides have been reported. Anthroquinone and quinone are produced on large scale by treatment of anthracene and phenol with chromic acid. A chromium III oxide coproduct is generated which is readily extracted into sulfuric acid.
Cr2 so4 3
Molar mass of Cr 2 SO 4 3 is Then, lookup atomic weights for each element in periodic table : Cr: Weights of atoms and isotopes are from NIST article. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students. By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy. How to cite? Enter a chemical formula to calculate its molar mass and elemental composition:. Computing molar mass molar weight To calculate molar mass of a chemical compound enter its formula and click 'Compute'. In chemical formula you may use: Any chemical element. Common compound names. Molar mass calculator also displays common compound name, Hill formula, elemental composition, mass percent composition, atomic percent compositions and allows to convert from weight to number of moles and vice versa. Computing molecular weight molecular mass To calculate molecular weight of a chemical compound enter it's formula, specify its isotope mass number after each element in square brackets. Examples of molecular weight computations: C[14]O[16]2 , S[34]O[16]2.
Gas laws. REL Recommended.
Then, lookup atomic weights for each element in periodic table : Cr: Weights of atoms and isotopes are from NIST article. WebQC is a web application with a mission to provide best-in-class chemistry tools and information to chemists and students. By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy. How to cite? Enter a chemical formula to calculate its molar mass and elemental composition:. Computing molar mass molar weight To calculate molar mass of a chemical compound enter its formula and click 'Compute'.
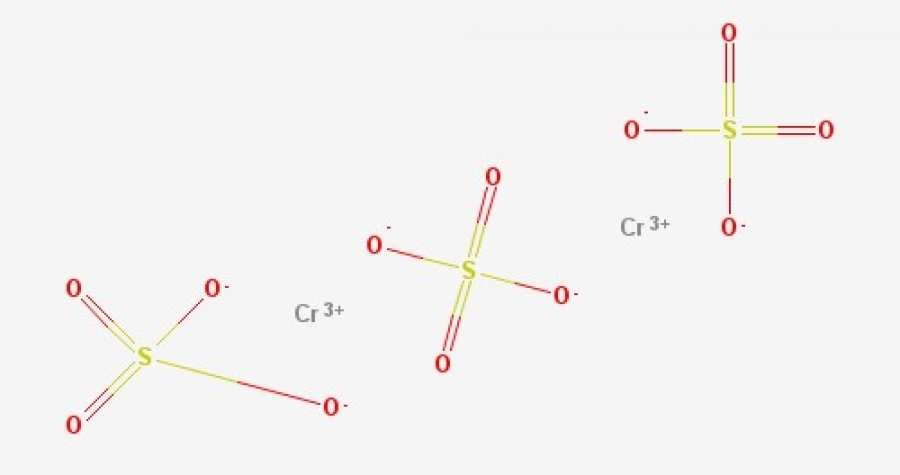
Moreover, it is a blue-grey solid that is soluble in water. While heating, it turns from blue color to green color. Let us learn chromium III sulfate formula here. Chromium III sulfate are usually those inorganic compounds that have formula i. These salts usually have either violet color or green color solids that are soluble when we put it inside the water. We use it commonly in tanning the leather material. Its chemical structure is present below in the form of a picture, in the common illustrations we basically use for the organic molecules. Image Source: PubChem.
Cr2 so4 3
This page looks at some aspects of chromium chemistry. It includes: reactions of chromium III ions in solution summarised from elsewhere on the site ; the interconversion of the various oxidation states of chromium; the chromate VI -dichromate VI equilibrium; and the use of dichromate VI ions as an oxidizing agent including titrations. The ion reacts with water molecules in the solution. A hydrogen ion is lost from one of the ligand water molecules:. The complex ion is acting as an acid by donating a hydrogen ion to water molecules in the solution. The water is, of course, acting as a base by accepting the hydrogen ion. Because of the confusing presence of water from two different sources the ligands and the solution , it is easier to simplify this:. However, if you write it like this, remember that the hydrogen ion isn't just falling off the complex ion. It is being pulled off by a water molecule in the solution.
7161 midland highway
Calculate molar mass of each element: multiply the atomic mass of each element by the number of atoms of that element in the compound. Retrieved Chemistry Electrochemistry Oxidation Numbers. Add them together: add the results from step 3 to get the total molar mass of the compound. Related: Molecular weights of amino acids. ISBN Add them together: add the results from step 3 to get the total molar mass of the compound. Computing molar mass molar weight To calculate molar mass of a chemical compound enter its formula and click 'Compute'. Add them together: add the results from step 3 to get the total molar mass of the compound. The molar mass of carbon dioxide is Molar mass of Cr 2 SO 4 3 is Chemical formula. How do oxidation numbers relate to valence electrons?
What is this information? Department of Transportation hazard labels, and a general description of the chemical.
How do oxidation numbers relate to valence electrons? PEL Permissible. By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy. TWA 0. By using this website, you signify your acceptance of Terms and Conditions and Privacy Policy. For example, water is H 2 O, meaning it contains two hydrogen atoms and one oxygen atom. Contact us. Cr CO 6. Related: Molecular weights of amino acids. Hazard statements.

In my opinion you have gone erroneous by.
I congratulate, magnificent idea and it is duly