Chlorine bohr model
This file contains additional information, probably added from the digital camera or scanner used to chlorine bohr model or digitize it. If the file has been modified from its original state, some details may not fully reflect the modified file.
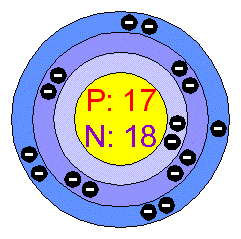
The two electrons in the first shell should be together not single. This is a Bohr model of a chlorine atom. Some Bohr models pair six of the seven electrons in the third valence shell. This helps to see that one valence electron is available for bonding. From my research, Bohr models of Cl either have the electrons in the second and third shells paired, or they don't have any paired electrons in the first, second, and third shells.
Chlorine bohr model
If you're seeing this message, it means we're having trouble loading external resources on our website. To log in and use all the features of Khan Academy, please enable JavaScript in your browser. Search for courses, skills, and videos. The Bohr model and atomic spectra. Learn how Bohr models are used to represent atoms. Atoms are way too small to see with the naked eye and even most microscopes. So, we represent atoms using models. Models help us visualize atomic structure. They also help us explain and predict the behavior of atoms. However, it's important to remember that no scientific model is perfect. Every model sacrifices some accuracy for simplicity, visibility, or usability. If a model was perfect, it wouldn't be a model—it would be the real thing! Atomic models are further complicated by quantum weirdness—electrons have both wave and particle properties.
Show preview Show formatting options Post answer.
We think you have liked this presentation. If you wish to download it, please recommend it to your friends in any social system. Share buttons are a little bit lower. Thank you! Published by Virginia McDaniel Modified over 5 years ago. They become negatively charged because there will be more electrons than protons.
General Education. Atoms are the basic building blocks of chemistry. They are the smallest unit which matter can be divided into without releasing electrically charged particles. Scientists have a number of ways for describing the structure of atoms. The Bohr Model is a modification of an earlier atomic model, the Rutherford Model.
Chlorine bohr model
Electric light bulbs contain a very thin wire in them that emits light when heated. The wire is called a filament. The particular wire used in light bulbs is made of tungsten. A wire made of any metal would emit light under these circumstances, but tungsten was chosen because the light it emits contains virtually every frequency and therefore, the light emitted by tungsten appears white. A wire made of some other element would emit light of some color that was not convenient for our uses. Every element emits light when energized by heating or passing electric current through it. Elements in solid form begin to glow when they are heated sufficiently, and elements in gaseous form emit light when electricity passes through them. This is the source of light emitted by neon signs and is also the source of light in a fire.
Fromis_9 reality
But what about elements with more electrons? The fuzzy electron cloud does a good job representing the wave nature of electrons. The properties of an element are determined by its outermost electrons, or those in the highest energy orbital. Commons is a freely licensed media file repository. Well, if you look at the atoms above chlorine, we see that all the atoms in a row have two shells. Lewis Symbols are simplified Bohr diagrams which only display electrons in the outermost energy level. Chapter 5: Ions and Ionic Compounds. This trend tells us the deeper into the rows you go the more shells you get. Electrons cannot exist at energies between these levels. Upload Log in. You can reuse this answer Creative Commons License. Want to join the conversation? However, since the rings are not intended to perfectly represent electron positions or energies, the exact spacing of the rings is not important. The following other wikis use this file: Usage on de. To use this website, you must agree to our Privacy Policy , including cookie policy.
Niels Bohr proposed an early model of the atom as a central nucleus containing protons and neutrons being orbited by electrons in shells. As previously discussed, there is a connection between the number of protons in an element, the atomic number that distinguishes one element from another, and the number of electrons it has. In all electrically-neutral atoms, the number of electrons is the same as the number of protons.
An atom may gain or lose electrons to achieve a full valence shell, the most stable electron configuration. Let's take a closer look at two atomic models, each with its own strengths and limitations. Their non-reactivity has resulted in their being named the inert gases or noble gases. Keep these things in mind when working with Bohr models:. Because the lower you get on the periodic table the more shells are added to store the extra electrons, Chlorine is in the 3rd row, so it has 3 shells. Ionatoms that has an electrical charge Ion: any atom or group of atoms that has an electrical charge. The darker the region, the more likely electrons are to be found there. Additional notes about Bohr models. And the ones above that row have only one shell. Search site Search Search. Until we measure an electron's position, we don't know exactly where it is. About project SlidePlayer Terms of Service.

Completely I share your opinion. In it something is also idea good, agree with you.
Paraphrase please the message