Chitinase
Federal government websites often end in.
Ayokunmi Oyeleye , Yahaya M. Normi; Chitinase: diversity, limitations, and trends in engineering for suitable applications. Chitinases catalyze the degradation of chitin, a ubiquitous polymer generated from the cell walls of fungi, shells of crustaceans, and cuticles of insects. They are gaining increasing attention in medicine, agriculture, food and drug industries, and environmental management. Their roles in the degradation of chitin for the production of industrially useful products and in the control of fungal pathogens and insect pests render them attractive for such purposes. However, chitinases have diverse sources, characteristics, and mechanisms of action that seem to restrain optimization procedures and render standardization techniques for enhanced practical applications complex.
Chitinase
Federal government websites often end in. The site is secure. Chitin, the second most abundant polysaccharide in nature after cellulose, is found in the exoskeleton of insects, fungi, yeast, and algae, and in the internal structures of other vertebrates. Chitinases are enzymes that degrade chitin. Chitinases contribute to the generation of carbon and nitrogen in the ecosystem. Chitin and chitinolytic enzymes are gaining importance for their biotechnological applications, especially the chitinases exploited in agriculture fields to control pathogens. Chitinases have a use in human health care, especially in human diseases like asthma. Chitinases have wide-ranging applications including the preparation of pharmaceutically important chitooligosaccharides and N-acetyl D glucosamine, preparation of single-cell protein, isolation of protoplasts from fungi and yeast, control of pathogenic fungi, treatment of chitinous waste, mosquito control and morphogenesis, etc. In this review, the various types of chitinases and the chitinases found in different organisms such as bacteria, plants, fungi, and mammals are discussed. Chitin and its associated materials have a broad usage in drug delivery, wound healing, dietary fiber, and in waste water treatment. These 2 forms of chitin vary in packing and polarities of adjacent chains in the succeeding sheets. The catabolism of chitin takes place in 2 steps, involving the initial cleavage of the chitin polymer by chitinases into chitin oligosaccharides and further cleavage to N-acetylglucosamine, and monosaccharides by chitobiases. Chitinases E. Chitinases have the ability to degrade chitin directly to low molecular weight chitooligomers, which serve a broad range of industrial, agricultural, and medical functions such as elicitor action and anti-tumor activity. Several pathogens contain chitin coats, giving them protection against both external and internal in a host environment.
In addition to the above studies, some comparative studies on S. The optimum temperature of chitinases obtained from other Serratia strains like S. Cloning, isolation, and Characterization of chitinase chitinase-producing bacterial strain UM01 Myxococcus fulvus J, chitinase.
Chitinases have the ability of chitin digestion that constitutes a main compound of the cell wall in many of the phytopathogens such as fungi. In the following investigation, a novel chitinase with antifungal activity was characterized from a native Serratia marcescens B4A. Partially purified enzyme had an apparent molecular mass of 54 kDa. Moreover, the Km and Vmax values for chitin were 8. Additionally, the effect of some cations and chemical compounds were found to stimulate the chitinase activity. In addition, Iodoacetamide and Idoacetic acid did not inhibit enzyme activity, indicating that cysteine residues are not part of the catalytic site of chitinase.
Chitinases are widely distributed enzymes and are present in a wide range of organisms including insects, plants, bacteria, fungi, and mammals. These enzymes play key roles in immunity, nutrition, pathogenicity, and arthropod molting. Human chitinases are reported to play a protective role against chitin-containing pathogens through their capability to degrade chitin present in the cell wall of pathogens. Now, human chitinases are gaining attention as the key players in innate immune response. Although the exact mechanism of their role in immune response is not known, studies in recent years begin to relate chitin recognition and degradation with the activation of signaling pathways involved in inflammation. The roles of both CHIT1 and AMCase in the development of various diseases have been revealed and several classes of inhibitors have been developed. However, a clear understanding could not be established due to complexities in the design of the right experiment for studying the role of human chitinase in various diseases.
Chitinase
This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:. Pear ring rot, a significant threat to pear production, is caused by Botryosphaeria dothidea, leveraging the complex dynamics of reactive oxygen species ROS during infection. Initially, plants employ their innate immune system, detecting pathogens through conserved molecular patterns and triggering a defense mechanism that includes ROS bursts, restricting pathogen growth. However, B. The current challenge lies in understanding how plants manage ROS levels to maintain resistance against B. In this study, researchers employed comparative transcriptome analysis to delve into the molecular dynamics of pear resistance to Botryosphaeria dothidea infection, pinpointing chitinase PbrChiAas a crucial regulator. By contrasting susceptible and resistant pear cultivars, the research unveiled that PbrChiA not only exhibits direct antifungal activity but also mitigates chitin-induced ROS accumulation by interacting with PbrLYK1b2, thereby enhancing pear's defense mechanisms. The analysis spanned early to later stages of infection , revealing a distinct upregulation of defense-related genes and pathways, such as photosynthesis inhibition and MAPK signaling, in the resistant cultivar, alongside heightened enzyme activities and reduced ROS levels, contributing to its resilience.
Budds subaru canada
The role of conserved tryptophan residues in the interaction of a bacterial cellulose binding domain with its ligand. Microbiol Implications of variations among chitinases for optimization and practical application. Parasitol Today. Xenorhabdus nematophila. Publisher: Portland Press Ltd. They have been studied for ages and there is an increasing knowledge about their enormous capabilities that is opening up the world of research in enzyme engineering for enhanced activities as well as for the development of new enzymatic functions. Correspondence to Dominik Hartl. Therefore, biological control strategy has become a vital advance in order to make sustainable agriculture possible. Chitin-containing waste can be used as a substrate shellfish chitin, shrimp cell waste, prawn cell waste, etc. Based on the gene sequence, it is reported that the cysteine-rich N-terminal appeared to be lost during evolution, as observed in one example of a class V chitinase, due to less selection pressure. Production, purification and properties of fungal chitinases—A review. Process Biochemistry J Microbiol. Plant Dis.
Federal government websites often end in. The site is secure.
Download PDF. Morimoto K. Find articles by Vikram Poria. This class consists of only exo-chitinases, and contains chitinases from plants, fungi, and bacteria. Chitinases E. This suggests that the CBD was necessary for complete degradation of insoluble crystalline chitin and not necessary for colloidal or soluble substrates. Colloidal chitin, chitin flakes, and other chitinous substrates, such as dimeric N-acetylglucosamine, are good inducers of chitinase. Variation in substrate specificity. Lv et al. Nucleic Acids Res. Nature

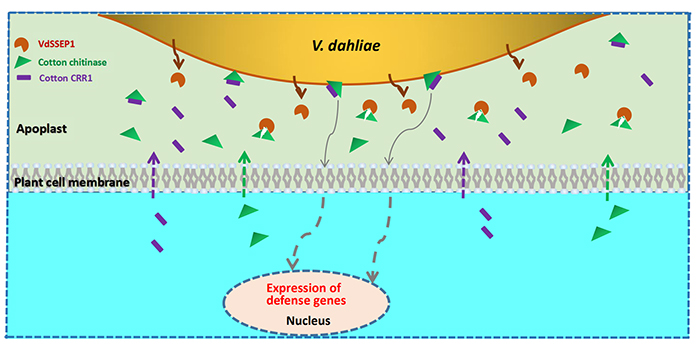
0 thoughts on “Chitinase”